Abstract
Objectives
Dramatic brain morphological changes occur throughout the third trimester of gestation. In this study, we investigated whether the predicted brain age (PBA) derived from graph convolutional network (GCN) that accounts for cortical morphometrics in third trimester is associated with postnatal abnormalities and neurodevelopmental outcome.
Methods
In total, 577 T1 MRI scans of preterm neonates from two different datasets were analyzed; the NEOCIVET pipeline generated cortical surfaces and morphological features, which were then fed to the GCN to predict brain age. The brain age index (BAI; PBA minus chronological age) was used to determine the relationships among preterm birth (i.e., birthweight and birth age), perinatal brain injuries, postnatal events/clinical conditions, BAI at postnatal scan, and neurodevelopmental scores at 30 months.
Results
Brain morphology and GCN-based age prediction of preterm neonates without brain lesions (mean absolute error [MAE]: 0.96 weeks) outperformed conventional machine learning methods using no topological information. Structural equation models (SEM) showed that BAI mediated the influence of preterm birth and postnatal clinical factors, but not perinatal brain injuries, on neurodevelopmental outcome at 30 months of age.
Conclusions
Brain morphology may be clinically meaningful in measuring brain age, as it relates to postnatal factors, and predicting neurodevelopmental outcome.
Clinical relevance statement
Understanding the neurodevelopmental trajectory of preterm neonates through the prediction of brain age using a graph convolutional neural network may allow for earlier detection of potential developmental abnormalities and improved interventions, consequently enhancing the prognosis and quality of life in this vulnerable population.
Key Points
•Brain age in preterm neonates predicted using a graph convolutional network with brain morphological changes mediates the pre-scan risk factors and post-scan neurodevelopmental outcomes.
•Predicted brain age oriented from conventional deep learning approaches, which indicates the neurodevelopmental status in neonates, shows a lack of sensitivity to perinatal risk factors and predicting neurodevelopmental outcomes.
•The new brain age index based on brain morphology and graph convolutional network enhances the accuracy and clinical interpretation of predicted brain age for neonates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predicted brain age (PBA) derived from neuroimaging and machine learning approaches has emerged as a biologically meaningful index that indicates the status of brain development or aging [1]. Estimation of neonatal brain age using the PBA measurement may be clinically useful in evaluating neurodevelopment and the effects of perinatal factors. Studies have demonstrated that brain structural and functional maturation is impaired by preterm birth, resulting in decreased cerebral volume [2], altered cortical surface area [3] and microstructural organization [4], and aberrant functional and structural connectivity [5, 6]. Moreover, various perinatal factors, such as birthweight, brain injuries, gestational age, and chronic lung disease of prematurity (CLD), also termed bronchopulmonary dysplasia, affect brain development and neurodevelopmental outcomes [7]. Thus, it remains clinically imperative to identify robust metrics that assess how various perinatal factors affect neurodevelopmental outcomes. PBA, with its increasing recognition in biological relevance [8, 9], may provide such clinical utility in assessing preterm brain development throughout the third trimester.
To derive the PBA measurement, studies to date have employed machine learning and deep learning (DL) methods [10, 11] that reveal important features without prior information or hypotheses. For instance, in aging brains, the PBA has been investigated using standard DL approaches [12] where an image volume is inputted into a convolutional neural network (CNN) and a number representing the whole brain age is outputted [13, 14]. However, these PBA models that were applied to structural connectivity data [15, 16] and myelin-based brain features [17] showed a lack of sensitivity in predicting neurodevelopmental outcomes [18].
Exploring specific morphological features could potentially boost the clinical significance of PBA, given that the third trimester is characterized by morphological changes that are highly sensitive to both age and pathology [19, 20]. Therefore, in contrast to previous brain age prediction strategies that directly applied DL models on MR images, we specifically focused on highly age- and pathology-related features during the third trimester, such as increases in cortical folding or volume, to enhance the clinical interpretation of neonatal brain age prediction. Additionally, numerous edges that connect neighboring vertices (i.e., points on the cortical surface) contain valuable topological information [21], which represents the location and adjacency among neighboring vertices. In this regard, a Graph Convolutional Network (GCN) model could be more suitable for the analysis of cortical morphological features, as it incorporates these topologies.
A brain age index (BAI; PBA minus chronological age) in neonates may indicate accelerated or delayed neurodevelopment. We hypothesize that the BAI at neonatal scan reflects the collective effects of pre-scan (pre- and postnatal) clinical factors, including preterm birth, perinatal brain injuries, postnatal treatments, neonatal infections, and postnatal cardiorespiratory complications. We also hypothesize that the BAI can predict neurodevelopmental outcomes. Furthermore, we hypothesize that these relationships with the BAI can only be uncovered when morphological features are effectively incorporated into the prediction model. Considering these, we explored the accuracy and clinical utility of PBA using a GCN model [22]. Cortical thickness, sulcal depth, and GM/WM intensity ratio [23] maps were extracted from the cortical mesh and inputted to the GCN. To evaluate the GCN-based PBA, we assessed (1) whether the PBA using GCN reflects the influence of pre-scan clinical factors on neurodevelopment; (2) whether BAI of neonatal MRI is a sensitive predictor of neurodevelopmental outcome at 30 months; (3) whether BAI at scan mediates the relationship between preterm birth-related clinical factors and neurodevelopmental outcome at 30 months; (4) whether the GCN approach outperforms other deep-learning brain age prediction algorithms that can clinically interpreting the developmental trajectories of preterm neonates.
Methods
Subjects
Our dataset comprised 129 preterm neonates (gestational age [GA] at birth range 24–33 weeks) admitted to UCSF (University of California at San Francisco) Benioff Children’s Hospital between June 2008 and May 2017 (Table 1) and 407 neonates from the developing Human Connectome Project (dHCP; http://www.developingconnectome.org/; GA at birth range 24–42 weeks; Table 2). Most subjects from UCSF were scanned twice, but some scans were excluded due to a large amount of motion artifact, resulting in a total of 170 MRI scans (mean postmenstrual age [PMA] range at 1st scan: 26.7–35.7 weeks; 2nd scan: 32.1–43.4 weeks). Parental consent was obtained through a protocol approved by the Institutional Committee on Human Research. In the dHCP cohort, the MRI images acquired from only singletons were included in this study. The images were visually inspected, and images with substantial motion on MRI or major focal parenchymal lesions at the time of their scan were excluded. The final dHCP sample consisted of 407 (187 female) neonates with a PMA of 29 to 45 weeks (mean PMA: 39.7 ± 3.1 weeks) in age at the time of the scan.
MRI acquisition and image processing
Newborns enrolled until 2011 (n = 56) were scanned on a 1.5-Tesla General Electric Signa HDxt system using a specialized high-sensitivity neonatal head coil built within a custom-built MRI-compatible incubator. T1-weighted images were acquired using sagittal 3-dimensional inversion recovery spoiled gradient echo (3D SPGR) (TR = 35; TE = 6; FOV = 256 × 192 mm2; number of excitations [NEX] = 1; and FA = 35°), yielding images with 1 × 1 × 1 mm3 resolution. Newborns enrolled between 2011 and 2017 (n = 73) were scanned on a 3-Tesla General Electric Discovery MR750 system. T1-weighted images were acquired using sagittal 3D IR-SPGR (inversion time = 450 ms; FOV = 180 × 180 mm2; NEX = 1; FA = 15°), yielding images with 0.7 × 0.7 × 1 mm3 resolution.
All T1 images from dHCP dataset were acquired on a Philips Achieva 3.0-T scanner using a 32-channel neonatal head coil [25]. All images were collected using an IR (inversion recovery) TSE sequence with the same resolution with TR = 4.8 s, TE = 8.7 ms, SENSE factor 2.26 (axial) and 2.66 (sagittal). Motion correction and super-resolution reconstruction techniques were employed, resulting in isotropic volumes of resolution \(0.5\times 0.5\times 0.5 \;{\mathrm{mm}}^{3}\). All images were collected as part of the dHCP and are described in detail in Makropoulos et al [26].
Cortical surfaces were constructed using NEOCIVET-v3 [27,28,29] (Methods S2). Key steps in the pipeline include image denoising, intensity nonuniformity correction, skull-striping, tissue segmentation, surface reconstruction, and surface registration. All major steps are specifically designed for neonatal MRI. Cortical morphology was quantitatively characterized by measuring cortical thickness, sulcal depth, and GM/WM intensity ratio [23] on the cortical surface at 81,924 vertices (163,840 polygons). Cortical features were harmonized using the ComBat method [30] prior to GCN training. A comparison of cortical features before and after the harmonization can be found in Figure S1. The validation of segmentation and surface construction quality using NEOCIVET is described in Figure S2.
Clinical factors and neurodevelopmental assessment
Neonatal demographic and clinical variables are described in Tables 1 and 2. Clinical variables are not publicly available for dHCP, so clinical analyses were only conducted on the UCSF dataset. Newborns with culture-positive sepsis, clinical signs of sepsis with negative blood culture, or meningitis were classified as having an infection. Newborns with clinical signs of patent ductus arteriosus (PDA; prolonged systolic murmur, bounding pulses, and hyperdynamic precordium) and evidence of left-to-right flow through the PDA on echocardiogram were classified as having a PDA. Necrotizing enterocolitis (NEC) was diagnosed according to Bell stage II criteria or higher.
To assess neonatal brain injuries, two pediatric neuroradiologists (A.J.B., H.J.L.) blinded to patient history reviewed individual MRI scans from UCSF and dHCP, including 3-D T1 and axial T2-weighted sequences. Severity of three leading drivers of neurodevelopmental deficits, i.e., intraventricular hemorrhage (IVH), ventriculomegaly (VM), and periventricular leukomalacia (PVL) or white matter injury, were visually scored (Methods S1). Details on brain injuries are listed in Tables S1-2. In the current study, we merged infants with mild injuries and those with no injury into one none-mild injury group, since the two groups exhibited no significant differences in the following analyses.
All the infants from UCSF were referred to the UCSF Intensive Care Nursery Follow-Up Program upon discharge for routine neurodevelopmental follow-up. Neurodevelopment was assessed using the Bayley-III, which was performed by unblinded clinicians at 30 months’ age corrected to 40 weeks, to assess cognitive, verbal/language and neuromotor performance. Follow-up was available in 38 of the 129 infants who survived to hospital discharge.
Brain age prediction
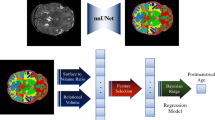
The proposed PBA model using GCN is illustrated in Fig. 1. GCNs [22] are designed to exploit the underlying graph structure of the data (Methods S3). We down-sampled 81,924 vertices on cortical surfaces to 1284 vertices using the icosahedron downsampling to investigate the prediction accuracy while saving computational time in training the GCN (Methods S4 and Figure S3). The input graphs combined the harmonized cortical features as the nodal features at 1284 vertices, with a sparse binary adjacency matrix representing the mesh topology, by which the edge in the graph was defined as the connections between each vertex and its neighbor vertices. GCNs consider spectral convolutions on graphs defined as the multiplication of a signal by a filter in the Fourier domain, which is approximated by Chebyshev polynomials. In the pooling operation, the vertices of the graph are rearranged to form a 1D pooling to ensure efficiency. The GCN used in this study consists of three convolutional layers and three pooling layers.
The proposed graph-based convolutional network for brain age prediction. Importantly, we did not use topology-varying surfaces because of the nature of the GCN model applied in this study. Rather, we considered cortical morphological changes that occur in relation to brain size and gyrification using cortical thickness, sulcal depth, and GM/WM intensity ratio. The GCN employed in our study requires identical graph/mesh structures for all individual inputs, while the features on nodes/vertices can vary
To compare our cortical surface-based GCN model with the conventional image-based DL model, we also built a PBA model by applying a CNN model [31] to T1 MR images directly (Methods S5). This model was fed a 3D scan as input and encoded each image slice using a 2D-CNN encoder. Next, it combines the slice encodings using an aggregation module, resulting in a single embedding for the scan. This model has been proven to be better than the conventional 3D-CNN-based model in adult brain age prediction study [31].
We split the data into k = 5 groups (folds), with 20% of data used for testing at each fold. The remaining 80% of the data were further iteratively split for training (64%) to fit the models and validation (16%) to tune the hyperparameters (in Methods S7 and Figure S4).
We added a bias correction as previously described in [12, 32] to correct age dependency of the training residuals. Briefly, we used a linear model \(PBA=\alpha *{PBA}^{\prime}+\beta\) to obtain an unbiased estimate of \(PBA\) as \({PBA}^{\prime}=(PBA-\beta )/\alpha\), where the parameters α and βare estimated during training (on both the combination of training and validation set) and are thus applied directly to the test set. After calculating PBA for each subject, we further calculated a metric, brain age index (BAI), that reflected a subject’s relative brain health status. BAI was measured by subtracting the postmenstrual age at the time of the MR scan from the unbiased \({\mathrm{PBA}}^{\prime}\) [14].
Statistical analysis
To validate our hypothesis that perinatal clinical factors negatively affect brain growth (lower BAI), each variable was dichotomized using clinically defined categorization or median if arbitrary (Table S1). Necrotizing enterocolitis was not included due to the small sample size (n = 5). We then tested the group difference in BAI for each variable separately in a univariate fashion while correcting for PMA at scan and other clinical factors, using a general linear mixed-effect model that addressed changes of within- and between-subject effects and removed the effects from covariates other than the main variable.
Moreover, we built structural equation models (SEM) that impute relationships between latent variables (Methods S9). Based on the hypothesized latent risk variables and timeline in Fig. 2, we analyzed multiple relationships/paths between severity of preterm birth, perinatal injuries, pre-scan postnatal factors, BAI at postnatal scan, and neurodevelopmental outcome scores at 30 months. This analysis, which was designed to identify the clinical variables and their paths leading to adverse neurodevelopmental outcomes, was conducted only on 50 MRI images from the UCSF dataset, including baseline and follow-up scans at 30 months from 38 preterm survivors.
We executed our GCN and CNN scripts with Pytorch 1.14.0. The SEM was executed using IBM SPSS Amos v24. Brain age bias correction and other statistics were performed using MATLAB 2021a. The brain age prediction code is available at https://github.com/bigting84/Brain-Age-Prediction. Anonymized data is available at https://github.com/bigting84/Neonatal-surface.
Results
Performance of GCN-based age prediction
We found that the GCN fed with the true brain mesh achieved an accuracy of prediction with a mean absolute error (MAE) of 0.963 weeks (5.3% of the total age range) and a correlation coefficient r of 0.94, which were smaller than the errors of the other two classic predictive models (random forest [RF] and general linear model [GLM]). Our model’s accuracy was also better than the CNN-based model (MAE = 1.05 weeks, r = 0.89) and the GCN on the random connections (MAE = 1.1 weeks, r = 0.89, Fig. 3).
A Comparison of brain age prediction errors among regression models. The height of each bar indicates the mean MAE, and the black line indicates the standard deviation of MAE per model. GCN demonstrates the best prediction results. B Scatter plot displaying PBA using GCN model vs. chronological brain age. We achieved a correlation coefficient r of 0.94 using GCN, which was significantly higher than the errors of the other models (GCN: MAE = 0.963 weeks, r = 0.94; CNN: MAE = 1.05 weeks, r = 0.89; GCN on the random connections: MAE = 1.1 weeks, r = 0.89; RF: MAE = 1.27 weeks, r = 0.85; GLM: MAE = 1.84 weeks, r = 0.75)
Clinical implications of the brain age index
Univariate analyses showed that various clinical variables were associated with lower brain age index (BAI; t > 3.1; p < 0.05, false discovery rate [FDR] corrected; Fig. 4A). Postnatal steroid exposure (p = 0.007, FDR corrected) presented the strongest association with lower BAI. Chronic lung disease (CLD; p = 0.031, FDR corrected) and birthweight lower than 1000 g (p = 0.021, FDR corrected) were also significantly associated with lower BAI. No significant association was found between the BAI estimated from the CNN model and any clinical variables (Fig. 4B).
Correlation of BAI with cognitive, language, or neuromotor scores in Bayley-III Scales (Fig. 5 left) showed that a lower BAI at neonatal scan was significantly associated with lower cognitive performance (r = 0.41; p = 0.0025, FDR corrected) and lower language performance (r = 0.27; p = 0.042, FDR corrected). No significant associations were found between the BAI estimated from the CNN model and developmental outcomes at 30 months (Fig. 5 right).
The outlined SEM is shown in Fig. 6 (with standardized estimates). The χ2 model-fit statistics indicated a significantly acceptable model fit (p < 0.001). BAI mediated the pathway from preterm birth to brain functional development at 30 months. BAI also mediated the pathway from postnatal factors to brain functional development at 30 months (p < 0.05). The relationship between brain injury and brain development at 30 months was not significant, for either the direct pathway or the pathway mediated by BAI (p < 0.05).
Results of path analysis. Rectangles represent manifest variables, and ellipses represent latent variables. Each single-headed arrow denotes a hypothesized unidirectional effect of one variable on another. Single-headed arrows represent the impact of one variable on another, and double-headed arrows represent covariances between pairs of variables. Numbers associated with effects are standardized regression coefficients. Asterisks refer to the paths that are statistically significant
Discussion
Clinical implications and applications
This study is the first to use structural equation modelling (SEM) to provide evidence of the temporal relationship among three stages of neurodevelopment: the context of preterm birth, postnatal brain development, and neurodevelopmental outcomes. That is, BAI at scan mediated the pathway from preterm birth and postnatal clinical factors, including birthweight, CLD, and exposure of postnatal steroids, to neurodevelopmental outcome at 30 months (Fig. 5). This suggests that brain morphological growth affected by preterm birth and postnatal factors is key to understanding later neurodevelopmental impairment. SEM did not provide sufficient evidence of a significant relationship between perinatal brain injuries and neurodevelopment outcomes. The underlying pathophysiology of how these clinical factors impair neurodevelopment may be attributed to both exogenous and endogenous factors that lead to brain insult from respiratory or circulatory insufficiency, hemorrhagic events, hypoxic-ischemic events, and inflammation [33,34,35,36,37].
Our results demonstrated that CLD has a strong association with impaired BAI. CLD primarily affects preterm infants who are exposed to prolonged mechanical ventilation and oxygen therapy for pulmonary complications [38, 39]. Chronic exposure to ventilation can result in oxygen toxicity, pulmonary inflammation, and lack of perfusion and oxygenation to the brain. Consequently, the immature brain becomes susceptible to hypoxia–ischemia, inflammation, germinal matrix injury, diffuse white matter injury, and diffuse gray matter injury [40, 41]. These complications, particularly diffuse white and gray matter lesions, contribute to neurodevelopmental impairment [42], consistent with the poor neurodevelopmental outcomes of CLD infants, including motor [43], language [44], and cognitive deficits [45,46,47].
Furthermore, our results revealed that postnatal exposure to steroids is associated with BAI impairment. Postnatal steroid therapy is traditionally provided to preterm neonates with BPD to reduce lung inflammation [48]. In our previous study, postnatal exposure to clinically routine doses of hydrocortisone or dexamethasone was associated with impaired cerebellar but not cerebral volumetric growth [49]. Using BAI based on cortical morphometrics and DL algorithms, however, we revealed additional adverse effects of postnatal glucocorticoids on cerebral growth. These results are consistent with previous findings demonstrating poor neurodevelopment following postnatal steroid exposure [50,51,52].
Brain age index as a potential predictor of cognitive and language development in preterm children
The clinical utility of BAIs is further supported by their relationships with neurodevelopmental outcomes at 30 months (Figure S8). BAI metric was not associated with neuromotor scores at 30 months. A possible explanation is that the morphology in the motor cortex, particularly cortical folding, was already relatively mature in our cohort, given folding forms earlier than the late 3rd trimester of gestation. Therefore, PBA based on cortical morphology extracted from these postnatal scans may not be sensitive to neuromotor impairment. Rather, previous studies showed that neuromotor impairment is associated with perinatal white matter brain injury [53, 54] and postnatal cerebellar growth [55].
Brain age prediction using GCN
By capturing age-related morphological alterations throughout the third trimester, we proposed that GCN-based deep learning (DL) with surface morphological features can better predict individual brain age and neurodevelopmental outcomes. To date, PBA was derived from machine learning methods fed with volumetric images directly [14] or structural connectome metrics [16]. However, image-fed CNNs may not be capable of detecting the morphology that varies along the cortical manifold, which appears to be a sensitive gauge of early neurodevelopment.
To address these limitations, we incorporated morphological features extracted from the cortical surface and surface topology into our GCN and found that its prediction accuracy is superior to state-of-the-art methods, including an image-fed CNN model. Our GCN model revealed the underlying association of brain maturation with clinical variables and developmental outcomes, whereas the CNN model did not, likely indicating that the CNN requires more samples for training to avoid overfitting and enhance prediction.
Limitations, future directions, and conclusions
Alterations in PBA could be a surrogate for brain developmental status. Notably, the brain age index is frequently influenced by various factors, giving rise to significant false positives and false negatives when assessing associations between BAI and other measures [56]. In this study, we only corrected the linear bias from the brain age index. As suggested by Smith et al [32], it is necessary to not only remove the linear dependency of brain age index on age but also the nonlinear dependence, especially the quadratic dependency of brain aging (as a function of age).
The data set used in this study was large but composed of heterogenous groups from two data sets, and the data from UCSF was acquired with different protocol/strength MRI. Although we performed harmonization, the number of population performed clinical evaluation and follow-up neurodevelopmental assessment were relatively small.
Further external validation will be performed in future studies. There is a huge time gap between the neonatal period and infancy at 30 months, which may limit the prediction ability and should be evaluated in a large sample study in the future.
It is important to approach SEM with a degree of caution, particularly when used for exploratory analyses. SEM is best employed when there are well-defined hypotheses about the relationships among variables. One of the limitations of SEM is that it assumes linearity and normality in the relationships among variables. It also requires a large sample size to ensure the stability and validity of its results. These are both marginally satisfied in this study. Furthermore, SEM’s capacity to provide plausible models does not prove that these models reflect true underlying processes. In light of these considerations, it would be prudent to moderate the claims made about SEM in this study.
Additional limitations in testing DL models in this study include the generalizability, and the interpretability of the model (including the use of uncertainty or confidence metrics) used in this study was not explicitly discussed, which will be guided by a checklist such as MAIC-10 in the future.
Despite these limitations, our study proposes a novel GCN that uses morphological features and topological patterns to predict brain age. The PBA, in turn, better explained neonatal developmental trajectory by linking pre-scan clinical factors and post-scan neurodevelopmental outcomes. Altogether, these findings provide the basis for future investigations aiming to extend the PBA measurement to practical clinical applications, such as the individualized prediction of neurodevelopmental outcomes.
Abbreviations
- BAI:
-
Brain age index
- CLD:
-
Chronic lung disease of prematurity
- CNN:
-
Convolutional neural network
- dHCP:
-
The developing Human Connectome Project
- DL:
-
Deep learning
- FDR:
-
False discovery rate
- GA:
-
Gestational age
- GCN:
-
Graph convolutional network
- GLM:
-
General linear model
- GM:
-
Gray matter
- IVH:
-
Intraventricular hemorrhage
- MAE:
-
Mean absolute error
- NEC:
-
Necrotizing enterocolitis
- PBA:
-
Predicted brain age
- PDA:
-
Patent ductus arteriosus
- PMA:
-
Postmenstrual age
- PVL:
-
Periventricular leukomalacia
- RF:
-
Random forest
- ROI:
-
Region of interest
- SEM:
-
Structural equation models
- VM:
-
Ventriculomegaly
- WM:
-
White matter
References
Jónsson BA, Bjornsdottir G, Thorgeirsson T et al (2019) Brain age prediction using deep learning uncovers associated sequence variants. Nat Commun 10:5409. https://doi.org/10.1038/s41467-019-13163-9
Ment LR, Vohr BR (2008) Preterm birth and the developing brain. Lancet Neurol 7:378–379. https://doi.org/10.1016/S1474-4422(08)70073-5
Ajayi-Obe M, Saeed N, Cowan F, Rutherford M, Edwards A (2000) Reduced development of cerebral cortex in extremely preterm infants. Lancet 356:1162–1163. https://doi.org/10.1016/s0140-6736(00)02761-6
Ball G, Srinivasan L, Aljabar P et al (2013) Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci U S A 110:9541–9546. https://doi.org/10.1073/pnas.1301652110
Pandit A, Robinson E, Aljabar P et al (2014) Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cereb Cortex 24:2324–2333. https://doi.org/10.1093/cercor/bht086
Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ (2016) Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb Cortex 26:322–333. https://doi.org/10.1093/cercor/bhu251
Ylijoki MK, Ekholm E, Ekblad M, Lehtonen L (2019) Prenatal risk factors for adverse developmental outcome in preterm infants-systematic review. Front Psychol 10:595. https://doi.org/10.3389/fpsyg.2019.00595
Franke K, Luders E, May A, Wilke M, Gaser C (2012) Brain maturation: predicting individual BrainAGE in children and adolescents using structural MRI. Neuroimage 63:1305–1312. https://doi.org/10.1016/j.neuroimage.2012.08.001
Cole JH, Franke K (2017) Predicting age using neuroimaging: innovative brain ageing biomarkers. Trends Neurosci 40:681–690. https://doi.org/10.1016/j.tins.2017.10.001
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521:436–444. https://doi.org/10.1038/nature14539
Peng H, Gong W, Beckmann CF, Vedaldi A, Smith SM (2019) Accurate brain age prediction with lightweight deep neural networks. Med Image Anal 68:101871. https://doi.org/10.1016/j.media.2020.101871
Ning K, Zhao L, Matloff W, Sun F, Toga AW (2020) Association of relative brain age with tobacco smoking, alcohol consumption, and genetic variants. Sci Rep 10:10. https://doi.org/10.1038/s41598-019-56089-4
Huang T-W, Chen H-T, Huang T-W et al (2017) Age estimation from brain MRI images using deep learning. In: 2017 IEEE 14th International symposium on biomedical imaging (ISBI 2017). IEEE, Melbourne, VIC, Australia, pp 849–852. https://doi.org/10.1109/ISBI.2017.7950650
Cole JH, Poudel RP, Tsagkrasoulis D et al (2017) Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage 163:115–124. https://doi.org/10.1016/j.neuroimage.2017.07.059
Kawahara J, Brown CJ, Miller SP et al (2017) BrainNetCNN: convolutional neural networks for brain networks; towards predicting neurodevelopment. Neuroimage 146:1038–1049. https://doi.org/10.1016/j.neuroimage.2016.09.046
Brown CJ, Moriarty KP, Miller SP et al (2017) Prediction of brain network age and factors of delayed maturation in very preterm infants. In: Medical image computing and computer assisted intervention − MICCAI 2017. Lecture notes in computer science, vol 10433. Springer, Cham. https://doi.org/10.1007/978-3-319-66182-7_10
Chen JV, Chaudhari G, Hess CP et al (2022) Deep learning to predict neonatal and infant brain age from myelination on brain MRI scans. Radiology 305:678–687. https://doi.org/10.1148/radiol.211860
He T, Kong R, Holmes A et al (2018) Is deep learning better than kernel regression for functional connectivity prediction of fluid intelligence? In: 2018 international workshop on pattern recognition in neuroimaging (PRNI). IEEE, Singapore, pp 1–4. https://doi.org/10.1109/PRNI.2018.8423958
Kim SY, Liu M, Hong S-J et al (2020) Disruption and compensation of sulcation-based covariance networks in neonatal brain growth after perinatal injury. Cereb Cortex 30:6238–6253. https://doi.org/10.1093/cercor/bhaa181
Liu M, Duffy BA, Sun Z et al (2020) Deep learning of cortical surface features using graph-convolution predicts neonatal brain age and neurodevelopmental outcome. 2020 IEEE 17th international symposium on biomedical imaging (ISBI). IEEE, pp 1335–1338. https://doi.org/10.1109/ISBI45749.2020.9098556
Kang SH, Liu M, Park G et al (2023) Different effects of cardiometabolic syndrome on brain age in relation to gender and ethnicity. Alzheimer’s research & therapy 15:1–10. https://doi.org/10.1186/s13195-023-01215-8
Defferrard M, Bresson X, Vandergheynst P (2016) Convolutional neural networks on graphs with fast localized spectral filtering. Advances in neural information processing systems 29. In: 30th Conference on neural information processing systems (NIPS 2016). NIPS, Barcelona, Spain. https://doi.org/10.5555/3157382.3157527
Lewis JD, Evans AC, Tohka J, Group BDC (2018) T1 white/gray contrast as a predictor of chronological age, and an index of cognitive performance. Neuroimage 173:341–350. https://doi.org/10.1016/j.neuroimage.2018.02.050
Kliegman RM, Hack M, Jones P, Fanaroff AA (1982) Epidemiologic study of necrotizing enterocolitis among low-birth-weight infants. Absence of identifiable risk factors. J Pediatr 100:440–444
Hughes EJ, Winchman T, Padormo F et al (2017) A dedicated neonatal brain imaging system. Magn Reson Med 78:794–804. https://doi.org/10.1002/mrm.26462
Makropoulos A, Robinson EC, Schuh A et al (2018) The developing human connectome project: a minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 173:88–112. https://doi.org/10.1016/j.neuroimage.2018.01.054
Kim H, Lepage C, Maheshwary R et al (2016) NEOCIVET: Towards accurate morphometry of neonatal gyrification and clinical applications in preterm newborns. Neuroimage 138:28–42. https://doi.org/10.1016/j.neuroimage.2016.05.034
Liu M, Lepage C, Jeon S et al (2019) A skeleton and deformation based model for neonatal pial surface reconstruction in preterm newborns. In: 2019 IEEE 16th International symposium on biomedical imaging (ISBI 2019). IEEE, Venice, Italy, pp 352–355. https://doi.org/10.1109/ISBI.2019.8759183
Liu M, Lepage C, Kim SY et al (2021) Robust cortical thickness morphometry of neonatal brain and systematic evaluation using multi-site MRI datasets. Front Neurosci 15:650082. https://doi.org/10.3389/fnins.2021.650082
Fortin JP, Cullen N, Sheline YI et al (2018) Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 167:104–120. https://doi.org/10.1016/j.neuroimage.2017.11.024
Gupta U, Lam PK, Steeg GV, Thompson PM (2021) Improved brain age estimation with slice-based set networks. In: 2021 IEEE 18th International symposium on biomedical imaging (ISBI). IEEE, Nice, France, pp 840–844. https://doi.org/10.1109/ISBI48211.2021.9434081
Smith SM, Vidaurre D, Alfaro-Almagro F, Nichols TE, Miller KL (2019) Estimation of brain age delta from brain imaging. Neuroimage 200:528–539. https://doi.org/10.1016/j.neuroimage.2019.06.017
Dempsey E, Barrington K (2007) Treating hypotension in the preterm infant: when and with what: a critical and systematic review. J Perinatol 27:469–478. https://doi.org/10.1038/sj.jp.7211774
Galinsky R, Lear CA, Dean JM et al (2018) Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Dev Med Child Neurol 60:126–133. https://doi.org/10.1111/dmcn.13629
Lemmers PM, Benders MJ, D'Ascenzo R et al (2016) Patent Ductus Arteriosus and Brain Volume. Pediatrics 137(4):e20153090. https://doi.org/10.1542/peds.2015-3090
van Vliet EO, de Kieviet JF, Oosterlaan J, van Elburg RM (2013) Perinatal infections and neurodevelopmental outcome in very preterm and very low-birth-weight infants: a meta-analysis. JAMA Pediatr 167:662–668. https://doi.org/10.1001/jamapediatrics.2013.1199
Zonnenberg I, van Dijk-Lokkart E, van Den Dungen F, Vermeulen R, van Weissenbruch M (2019) Neurodevelopmental outcome at 2 years of age in preterm infants with late-onset sepsis. Eur J Pediatr 178:673–680. https://doi.org/10.1007/s00431-019-03339-2
Gallini F, Coppola M, Umberto De Rose D et al (2021) Neurodevelopmental outcomes in very preterm infants. The role of severity of Bronchopulmonary Dysplasia. Early Hum Dev 152:105275. https://doi.org/10.1016/j.earlhumdev.2020.105275
Kinsella JP, Greenough A, Abman SH (2006) Bronchopulmonary dysplasia. Lancet 367:1421–1431. https://doi.org/10.1016/S0140-6736(06)68615-7
Albertine KH (2012) Brain injury in chronically ventilated preterm neonates: collateral damage related to ventilation strategy. Clin Perinatol 39:727–740. https://doi.org/10.1016/j.clp.2012.06.017
Malavolti AM, Bassler D, Arlettaz-Mieth R et al ( 2018) Bronchopulmonary dysplasia—impact of severity and timing of diagnosis on neurodevelopment of preterm infants: a retrospective cohort study. BMJ Paediatrics Open 2:e000165. https://doi.org/10.1136/bmjpo-2017-000165
Lean RE, Han RH, Smyser TA et al (2019) Altered neonatal white and gray matter microstructure is associated with neurodevelopmental impairments in very preterm infants with high-grade brain injury. Pediatr Res 86:365–374. https://doi.org/10.1038/s41390-019-0461-1
Weeke LC, Groenendaal F, Mudigonda K et al (2018) A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr 192(33–40):e32. https://doi.org/10.1016/j.jpeds.2017.09.043
Singer LT, Siegel AC, Lewis B, Hawkins S, Yamashita T, Baley J (2001) Preschool language outcomes of children with history of bronchopulmonary dysplasia and very low birth weight. J Dev Behav Pediatr 22:19–26. https://doi.org/10.1097/00004703-200102000-00003
Natarajan G, Pappas A, Shankaran S et al (2012) Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev 88:509–515. https://doi.org/10.1016/j.earlhumdev.2011.12.013
Short EJ, Klein NK, Lewis BA et al (2003) Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics 112:e359–e359. https://doi.org/10.1542/peds.112.5.e359
Singer L, Yamashita T, Lilien L, Collin M, Baley J (1997) A longitudinal study of developmental outcome of infants with bronchopulmonary dysplasia and very low birth weight. Pediatrics 100:987–993. https://doi.org/10.1542/peds.100.6.987
Malavolti AM, Bassler D, Arlettaz-Mieth R et al (2018) Bronchopulmonary dysplasia—impact of severity and timing of diagnosis on neurodevelopment of preterm infants: a retrospective cohort study. BMJ Paediatrics Open 2:e000165. https://doi.org/10.1136/bmjpo-2017-000165
Tam EW, Chau V, Ferriero DM et al (2011) Preterm Cerebellar Growth Impairment After Postnatal Exposure to Glucocorticoids. Sci Transl Med 3:105ra105-105ra105. https://doi.org/10.1126/scitranslmed.3002884
Cheong JLY, Doyle LW (2019) Long-term effects of postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Balancing the risks and benefits. Semin Fetal Neonatal Med 24(3):197–201. https://doi.org/10.1016/j.siny.2019.03.002
Jobe AH (2004) Postnatal corticosteroids for preterm infants–do what we say, not what we do. New Eng J Med 350:1349–1350. https://doi.org/10.1056/NEJMe048031
Doyle LW (2021) Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Neonatology 118:244–251. https://doi.org/10.1159/000515950
Guo T, Duerden EG, Adams E et al (2017) Quantitative assessment of white matter injury in preterm neonates: association with outcomes. Neurology 88:614–622. https://doi.org/10.1212/wnl.0000000000003606
Saha S, Pagnozzi A, Bourgeat P et al (2020) Predicting motor outcome in preterm infants from very early brain diffusion MRI using a deep learning convolutional neural network (CNN) model. Neuroimage 215:116807. https://doi.org/10.1016/j.neuroimage.2020.116807
Messerschmidt A, Prayer D, Brugger PC et al (2008) Preterm birth and disruptive cerebellar development: assessment of perinatal risk factors. Eur J Paediatr Neurol 12:455–460. https://doi.org/10.1016/j.ejpn.2007.11.003
Le TT, Kuplicki RT, McKinney BA, Yeh HW, Thompson WK, Paulus MP (2018) A nonlinear simulation framework supports adjusting for age when analyzing BrainAGE. Front Aging Neurosci 10:317. https://doi.org/10.3389/fnagi.2018.00317
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This study was supported by the National Institutes of Health grants (P50NS035902, P01NS082330, R01NS046432, R01HD072074; P41EB015922; U54EB020406; U19AG024904; U01NS086090; 003585–00001). HK was funded by the BrightFocus Foundation Award (A2019052S).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hosung Kim.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors, Mengting Liu, has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study. Parental consent was obtained through a protocol approved by the Institutional Committee on Human Research.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in the following:
Paper 1: “Hippocampal asymmetry of regional development and structural covariance in preterm neonates,” and Paper 2: “Disruption and compensation of sulcation-based covariance networks in neonatal brain growth after perinatal injury.”
The topic of Paper 1 is how preterm birth may exert an impact on hippocampal development in neonates, while Paper 2 is about how brain injury in preterm birth may alter the topological organization of brain structural network in neonates. Our current study, however, focuses on brain age prediction in neonates, which is a completely different research topic compared to the previously published work.
The manuscript has a pre-print https://doi.org/10.1101/2021.05.15.444320
Methodology
• retrospective
• cross-sectional study
• multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. Liu and M. Lu contributed equally to this work.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, M., Lu, M., Kim, S.Y. et al. Brain age predicted using graph convolutional neural network explains neurodevelopmental trajectory in preterm neonates. Eur Radiol 34, 3601–3611 (2024). https://doi.org/10.1007/s00330-023-10414-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10414-8