Abstract
Objectives
The purpose of this study was to evaluate a three-material decomposition algorithm for hepatic fat quantification using a dual-layer computed tomography (DL-CT) and MRI as reference standard on a large patient cohort.
Method
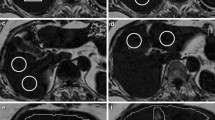
A total of 104 patients were retrospectively included in our study, i.e., each patient had an MRI exam and a DL-CT exam in our institution within a maximum of 31 days. Four regions of interest (ROIs) were positioned blindly and similarly in the liver, by two independent readers on DL-CT and MRI images. For DL-CT exams, all imaging phases were included. Fat fraction agreement between CT and MRI was performed using intraclass correlation coefficients (ICC), determination coefficients R2, and Bland–Altman plots. Diagnostic performance was determined using sensitivity, specificity, and positive and negative predictive values. The cutoff for steatosis was 5%.
Results
Correlation between MRI and CT data was excellent for all perfusion phases with ICC calculated at 0.99 for each phase. Determination coefficients R2 were also good for all perfusion phases (about 0.95 for all phases). Performance of DL-CT in the diagnosis of hepatic steatosis was good with sensitivity between 89 and 91% and specificity ranging from 75 to 80%, depending on the perfusion phase. The positive predictive value was ranging from 78 to 93% and the negative predictive value from 82 to 86%.
Conclusion
Multi-material decomposition in DL-CT allows quantification of hepatic fat fraction with a good correlation to MRI data.
Clinical relevance statement
The use of DL-CT allows for detection of hepatic steatosis. This is especially interesting as an opportunistic finding CT performed for other reasons, as early detection can help prevent or slowdown the development of liver metabolic disease.
Key Points
• Hepatic fat fractions provided by the dual-layer CT algorithm is strongly correlated with that measured on MRI.
• Dual-layer CT is accurate to detect hepatic steatosis ≥ 5%.
• Dual-layer CT allows opportunistic detection of steatosis, on CT scan performed for various indications.






Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- DE-CT:
-
Dual-energy computed tomography
- DL-CT:
-
Dual-layer computed tomography
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- VNC:
-
Virtual non-contrast
References
Cotter TG, Rinella M (2020) Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 158(7):1851–1864
Huang TD, Behary J, Zekry A (2020) Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern Med J. 50(9):1038–47
Jennison E, Patel J, Scorletti E, Byrne CD (2019) Diagnosis and management of non-alcoholic fatty liver disease. Postgrad Med J 95(1124):314–322
Chalasani N, Younossi Z, Lavine JE et al (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1):328–357
Yokoo T, Bydder M, Hamilton G et al (2009) Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 251(1):67–76
Caussy C, Reeder SB, Sirlin CB, Loomba R (2018) Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 68(2):763–772
Kukuk GM, Hittatiya K, Sprinkart AM et al (2015) Comparison between modified Dixon MRI techniques, MR spectroscopic relaxometry, and different histologic quantification methods in the assessment of hepatic steatosis. Eur Radiol 25(10):2869–2879
Flohr TG, McCollough CH, Bruder H et al (2006) First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol 16(2):256–268
Hamid S, Nasir MU, So A, Andrews G, Nicolaou S, Qamar SR (2021) Clinical applications of dual-energy CT. Korean J Radiol 22(6):970–982
Hur BY, Lee JM, Hyunsik W et al (2014) Quantification of the fat fraction in the liver using dual-energy computed tomography and multimaterial decomposition. J Comput Assist Tomogr 38(6):845–852
Gassenmaier S, Kähm K, Walter SS, Machann J, Nikolaou K, Bongers MN (2021) Quantification of liver and muscular fat using contrast-enhanced dual source dual energy computed tomography compared to an established multi-echo Dixon MRI sequence. Eur J Radiol 142:109845
Molwitz I, Campbell GM, Yamamura J et al (2022) Fat quantification in dual-layer detector spectral computed tomography: experimental development and first in-patient validation. Invest Radiol 57(7):463–469
Molwitz I, Leiderer M, Özden C, Yamamura J (2020) Dual-energy computed tomography for fat quantification in the liver and bone marrow: a literature review. Rofo 192(12):1137–1153
Corrias G, Erta M, Sini M et al (2021) Comparison of multimaterial decomposition fat fraction with DECT and proton density fat fraction with IDEAL IQ MRI for quantification of liver steatosis in a population exposed to chemotherapy. Dose Response 19(2):1559325820984938
Zhang Q, Zhao Y, Wu J et al (2021) Quantification of hepatic fat fraction in patients with non alcoholic fatty liver disease: comparison of multimaterial decomposition algorithm and fat (water)-based material decomposition algorithm using single-source dual-energy computed tomography. J Comput Assist Tomogr 45(1):12–17
Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F (2009) Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 51(3):433–445
Zhang PP, Choi H, Ohliger M (2022) Detection of fatty liver using a virtual non-contrast dual-energy CT. Abdom Radiol (NY) 47(6):2046–2056
Xu J, Boesen MR, Lindskov Hansen S et al (2022) Assessment of liver fat: dual-energy CT versus conventional CT with and without contrast. Diagnostics 12(3):708
Hyung Choi M, Lee YJ, Jeong Y et al (2021) Dual-energy CT of the liver: true noncontrast vs. virtual noncontrast images derived from multiple phases for the diagnosis of fatty liver. Eur J Radiol. 140:109741
Cao Q, Yan C, Han X et al (2022) Quantitative evaluation of nonalcoholic fatty liver in rat by material decomposition techniques using rapid-switching dual energy CT. Acad Radiol 29(6):e91-e97
Hong SB, Lee NK, Kim S et al (2022) Hepatic fat quantification with the multi-material decomposition algorithm by using low-dose non-contrast material-enhanced dual-energy computed tomography in a prospectively enrolled cohort. Medicina (Kaunas) 58(10):1459
Rassouli N, Etesami M, Dhanantwari A, Rajiah P (2017) Detector-based spectral CT with a novel dual-layer technology: principles and applications. Insights Imaging 8(6):589–598
Mignot A, Ayav A, Quillot D et al (2017) Extensive lymph node dissection during pancreaticoduodenectomy: a risk factor for hepatic steatosis? Abdom Radiol (NY) 42(7):1880–1887
Alvarez RE, Macovski A (1976) Energy-selective reconstructions in X-ray computerised tomography. Phys Med Biol 21(5):733–744
Mendonca PRS, Lamb P, Sahani DV (2014) A flexible method for multi-material decomposition of dual-energy CT images. IEEE Trans Med Imaging 33(1):99–116
Idilman IS, Ozdeniz I, Karcaaltincaba M (2016) Hepatic steatosis: etiology, patterns, and quantification. Semin Ultrasound CT MRI 37(6):501–510
Patel J, Bettencourt R, Cui J et al (2016) Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Ther Adv Gastroenterol 9(5):692–701
Le TA, Chen J, Changchien C et al (2012) Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 56(3):922–932
Ma J, Song ZQ, Yan FH (2014) Separation of hepatic iron and fat by dual-source dual-energy computed tomography based on material decomposition: an animal study. PLoS One 9(10):e110964
Fischer MA, Reiner CS, Raptis D et al (2011) Quantification of liver iron content with CT-added value of dual-energy. Eur Radiol 21(8):1727–1732
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr Mathilde Vermersch.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
Benjamin Robert with Philips Healthcare as Clinical Scientist.
Garit Kafri and Eran Langzam as Clinical Scientists at Philips Healthcare. They both developed the software for intrahepatic fat quantification but did not have access to patient data and intrahepatic fat measurements were performed blind to the gold standard.
The other authors of this manuscript declare no relationships with any companies.
Statistics and biometry
One of the authors has significant statistical expertise (Dr Mathilde Vermersch).
No complex statistical methods were necessary for this paper.
Informed consent
This study met the criteria of non-interventional, retrospective research and was approved by international review board (CERIM) (IRB CRM 2201-222). Inform consent for all patients was waived by IRB.
Ethical approval
Institutional Review Board approval was obtained: IRB CRM 2201–222.
Methodology
• retrospective
• observational
• performed at one institution at CHU de Lille
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demondion, E., Ernst, O., Louvet, A. et al. Hepatic fat quantification in dual-layer computed tomography using a three-material decomposition algorithm. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10382-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10382-z