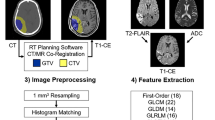
Abstract
Objectives
As a few types of glioma, young high-risk low-grade gliomas (HRLGGs) have higher requirements for postoperative quality of life. Although adjuvant chemotherapy with delayed radiotherapy is the first treatment strategy for HRLGGs, not all HRLGGs benefit from it. Accurate assessment of chemosensitivity in HRLGGs is vital for making treatment choices. This study developed a multimodal fusion radiomics (MFR) model to support radiochemotherapy decision-making for HRLGGs.
Methods
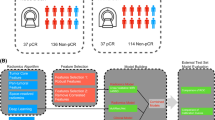
A MFR model combining macroscopic MRI and microscopic pathological images was proposed. Multiscale features including macroscopic tumor structure and microscopic histological layer and nuclear information were grabbed by unique paradigm, respectively. Then, these features were adaptively incorporated into the MFR model through attention mechanism to predict the chemosensitivity of temozolomide (TMZ) by means of objective response rate and progression free survival (PFS).
Results
Macroscopic tumor texture complexity and microscopic nuclear size showed significant statistical differences (p < 0.001) between sensitivity and insensitivity groups. The MFR model achieved stable prediction results, with an area under the curve of 0.950 (95% CI: 0.942–0.958), sensitivity of 0.833 (95% CI: 0.780–0.848), specificity of 0.929 (95% CI: 0.914–0.936), positive predictive value of 0.833 (95% CI: 0.811–0.860), and negative predictive value of 0.929 (95% CI: 0.914–0.934). The predictive efficacy of MFR was significantly higher than that of the reported molecular markers (p < 0.001). MFR was also demonstrated to be a predictor of PFS.
Conclusions
A MFR model including radiomics and pathological features predicts accurately the response postoperative TMZ treatment.
Clinical relevance statement
Our MFR model could identify young high-risk low-grade glioma patients who can have the most benefit from postoperative upfront temozolomide (TMZ) treatment.
Key Points
• Multimodal radiomics is proposed to support the radiochemotherapy of glioma.
• Some macro and micro image markers related to tumor chemotherapy sensitivity are revealed.
• The proposed model surpasses reported molecular markers, with a promising area under the curve (AUC) of 0.95.






Similar content being viewed by others
Abbreviations
- AFMF:
-
Attention-based feature map fusion block
- AUC:
-
Area under curve
- CR:
-
Complete response
- CT:
-
Computed tomography
- HRLGG:
-
High-risk low-grade glioma
- KM:
-
Kaplan and Meier
- MFR:
-
Multimodal fusion radiomics
- MGMT:
-
Methylguanine-DNA-methyltransferase
- MR:
-
Minor response
- MRI:
-
Magnetic resonance imaging
- PD:
-
Progression disease
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- RANO:
-
Response Assessment in Neuro-Oncology
- ROI:
-
Region of interest
- SD:
-
Stable disease
- TMZ:
-
Temozolomide
References
Dirven L, Reijneveld JC, Taphoorn MJB et al (2019) Impact of radiation target volume on health-related quality of life in patients with low-grade glioma in the 2-year period post treatment: a secondary analysis of the eortc 22033–26033. Int J Radiat Oncol 104:90–100
Tong S, Wang Y, Wu J, Long J, Zhong P, Wang B (2021) Comprehensive pharmacogenomics characterization of temozolomide response in gliomas. Eur J Pharm 912:174580
Klein M, Drijver AJ, van den Bent MJ et al (2021) Memory in low-grade glioma patients treated with radiotherapy or temozolomide: a correlative analysis of EORTC study 22033–26033. Neuro Oncol 23:803–811
van den Bent MJ, Afra D, De Witte O et al (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366:985–990
Douw L, Klein M, Fagel SS et al (2009) Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 8:810–818
Buckner JC, Pugh SL, Shaw EG et al (2014) Phase III study of radiation therapy (RT) with or without procarbazine, CCNU, and vincristine (PCV) in low-grade glioma: RTOG 9802 with Alliance, ECOG, and SWOG. J Clin Oncol. https://doi.org/10.1200/jco.2014.32.15_suppl.2000
Rudà R, Pellerino A, Pace A et al (2019) Efficacy of initial temozolomide for high-risk low grade gliomas in a phase II AINO (Italian Association for Neuro-oncology) study: a post-hoc analysis within molecular subgroups of who 2016. J Neuro Onco 145:115–123
Wick W, Roth P, Hartmann C et al (2016) Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol 18:1529–1537
McNamara MG, Jiang H, Lim-Fat MJ et al (2017) Treatment outcomes in 1p19q co-deleted/partially deleted gliomas. Can J Neurol Sci 44:288–294
Brigitta GB, Monika EH, van den Bent MJ et al (2016) Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 17:1521–1532
Malmström A, Grønberg BH, Marosi C et al (2012) Temozolomide 364 versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 365 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13:916–926
Yu J, Shi Z, Lian Y et al (2017) Noninvasive IDH1 mutation estimation based on a quantitative radiomics approach for grade II glioma. Eur Radiol 27:3509–3522
Wu G, Chen Y, Wang Y et al (2017) Sparse representation-based radiomics for the diagnosis of brain tumors. IEEE Trans Med Imaging 37:893–905
Wu G, Shi Z, Chen Y et al (2019) A sparse representation-based radiomics for outcome prediction of higher grade gliomas. Med Phys 46:250–261
Macyszyn L, Akbari H, Pisapia JM et al (2016) Imaging patterns predict patient survival and molecular subtype in glioblastoma via machine learning techniques. Neuro Oncol 18:417–425
Baid U, Rane SU, Talbar S et al (2020) Overall survival prediction in glioblastoma with radiomic features using machine learning. Front Comput Neurosci 14:1–9
Choi YS, Bae S, Chang JH et al (2021) Fully automated hybrid approach to predict the IDH mutation status of gliomas via deep learning and radiomics. Neuro Oncol 23:304–313
Sun P, Wang D, Mok VC, Shi L (2019) Comparison of feature selection methods and machine learning classifiers for radiomics analysis in glioma grading. IEEE Access 7:102010–102020
Aerts HJWL, Velazquez ER, Leijenaar RTH et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:1–9
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Jin L, Shi F, Chun Q et al (2021) Artificial intelligence neuropathologist for glioma classification using deep learning on hematoxylin and eosin stained slide images and molecular markers. Neuro Oncol 23:44–52
Iftikhar MA, Rathore S, Nasrallah M (2019) Analysis of microscopic images via deep neural networks can predict outcome and IDH and 1p/19q codeletion status in gliomas. J Neuropathol Exp Neurol 78:553
Mobadersany P, Yousefi S, Amgad M et al (2018) Predicting cancer outcomes from histology and genomics using convolutional networks. Proc Natl Acad Sci 115:E2970–E2979
Lipkova J, Chen RJ, Chen B et al (2022) Artificial intelligence for multimodal data integration in oncology. Cancer Cell 40:1095–1110
Sleeman WC, Kapoor R, Ghosh P (2022) Multimodal classification: current landscape, taxonomy and future directions. ACM Comput Surv 55:150
Hsu WW, Guo JM, Pei L et al (2022) A weakly supervised deep learning-based method for glioma subtype classification using WSI and mpMRIs. Sci Rep 12:6111
Rathore FA, Khan HS, Ali HM et al (2022) Survival prediction of glioma patients from integrated radiology and pathology images using machine learning ensemble regression methods. Appl Sci 12:10357
Vallières M, Freeman CR, Skamene SR, El Naqa I (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. https://doi.org/10.1088/0031-9155/60/14/5471
Zhang H, Wu C, Zhang Z et al (2020) Resnest: split-attention networks. In: IEEE/CVF Conference on CVPR, pp 417–428
Li X, Wang W, Hu X, Yang J (2019) Selective kernel networks. In: IEEE/CVF Conference on CVPR, pp 510–519
Guo P, Banerjee K, Stanley RJ et al (2015) Nuclei-based features for uterine cervical cancer histology image analysis with fusion-based classification. IEEE J Biomed Health Inform 20:1595–1607
Chen C, Wang Y, Niu J, Liu X, Li Q, Gong X (2021) Domain knowledge powered deep learning for breast cancer diagnosis based on contrast-enhanced ultrasound videos. IEEE Trans Med Imaging 40:2439–2451
Yu J, Deng Y, Liu T et al (2020) Lymph node metastasis prediction of papillary thyroid carcinoma based on transfer learning radiomics. Nat Commun 11:1–10
Ceccarelli M, Barthel FP, Malta TM et al (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164:550–563
Zheng X, Yao Z, Huang Y et al (2020) Deep learning radiomics can predict axillary lymph node status in early-stage breast cancer. Nat Commun 11:1–9
Chougule T, Gupta RK, Saini J et al (2021) Radiomics signature for temporal evolution and recurrence patterns of glioblastoma using multimodal magnetic resonance imaging. NMR Biomed 35:e4647
Li Z, Yan J, Zhang S et al (2022) Glioma survival prediction from whole-brain MRI without tumor segmentation using deep attention network: a multicenter study. Eur Radiol 32:5719–5729
Talo M, Yildirim O, Baloglu UB, Aydin G, Acharya U (2019) Convolutional neural networks for multi-class brain disease detection using MRI images. Comput Med Imag Grap 78:101673
Lu Z, Bai Y, Chen Y et al (2020) The classification of gliomas based on a Pyramid dilated convolution resnet model. Pattern Recogn Lett 133:173–179
Funding
This study has received funding by the National Natural Science Foundation of China Youth Fund (No. 62001119), the Major research plan of the National Natural Science Foundation (No. 91959127) and the Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01) and ZJLab.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
Jinhua Yu, School of Information Science and Technology, Fudan University, No. 220 Handan Rd, 200433, Shanghai, China.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Jinhua Yu has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained. The study was approved by the Ethics Committee of the Huashan Hospital and the Shanghai International Hospital.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, G., Shi, Z., Li, Z. et al. Study of radiochemotherapy decision-making for young high-risk low-grade glioma patients using a macroscopic and microscopic combined radiomics model. Eur Radiol 34, 2861–2872 (2024). https://doi.org/10.1007/s00330-023-10378-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10378-9