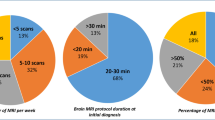
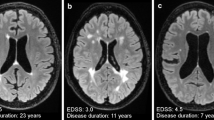
Abstract
Magnetic resonance imaging (MRI) is the most sensitive technique for detecting inflammatory demyelinating lesions in multiple sclerosis (MS) and plays a crucial role in diagnosis and monitoring treatment effectiveness, and for predicting the disease course. In clinical practice, detection of MS lesions is mainly based on T2-weighted and contrast-enhanced T1-weighted sequences. Contrast-enhancing lesions (CEL) on T1-weighted sequences are related to (sub)acute inflammation, while new or enlarging T2 lesions reflect the permanent footprint from a previous acute inflammatory demyelinating event. These two types of MRI features provide redundant information, at least in regular monitoring of the disease. Due to the concern of gadolinium deposition after repetitive injections of gadolinium-based contrast agents (GBCAs), scientific organizations and regulatory agencies in Europe and North America have proposed that these contrast agents should be administered only if clinically necessary. In this article, we provide data on the mode of action of GBCAs in MS, the indications of the use of these agents in clinical practice, their value in MS for diagnostic, prognostic, and monitoring purposes, and their use in specific populations (children, pregnant women, and breast-feeders). We discuss imaging strategies that achieve the highest sensitivity for detecting CELs in compliance with the safety regulations established by different regulatory agencies. Finally, we will briefly discuss some alternatives to the use of GBCA for detecting blood–brain barrier disruption in MS lesions.
Clinical relevance statement
Although use of GBCA at diagnostic workup of suspected MS is highly valuable for diagnostic and prognostic purposes, their use in routine monitoring is not mandatory and must be reduced, as detection of disease activity can be based on the identification of new or enlarging lesions on T2-weighted images.
Key Points
• Both the EMA and the FDA state that the use of GBCA in medicine should be restricted to clinical scenarios in which the additional information offered by the contrast agent is required.
• The use of GBCA is generally recommended in the diagnostic workup in subjects with suspected MS and is generally not necessary for routine monitoring in clinical practice.
• Alternative MRI-based approaches for detecting acute focal inflammatory MS lesions are not yet ready to be used in clinical practice.
Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CEL:
-
Contrast-enhancing lesion
- CMSC:
-
Consortium of Multiple Sclerosis Centers
- DMT:
-
Disease-modifying treatment
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- EMA:
-
European Medicines Agency
- EPI:
-
Echo planar imaging
- ESMRMB:
-
European Society for Magnetic Resonance in Medicine and Biology
- ESNR:
-
European Society of Neuroradiology
- FA:
-
Fractional anisotropy
- FDA:
-
Food and Drug Administration
- FLAIR:
-
Fluid-attenuated inversion recovery
- GBCA:
-
Gadolinium-based contrast agent
- GRE:
-
Gradient-recalled echo
- GREC:
-
Gadolinium Research & Education Committee
- MAGNIMS:
-
Magnetic resonance imaging in MS
- MRI:
-
Magnetic resonance imaging
- MS:
-
Multiple sclerosis
- NAIMS:
-
North American Imaging in Multiple Sclerosis
- PML:
-
Progressive multifocal leukoencephalopathy
- SE:
-
Spin-echo
- T1w:
-
T1-weighted
- T2w:
-
T2-weighted
- TSE/FSE:
-
Turbo/fast spin-echo
References
Wattjes MP, Ciccarelli O, Reich DS et al (2021) 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol 20:653–670
Rovira A, Auger C, Alonso J (2013) Magnetic resonance monitoring of lesion evolution in multiple sclerosis. Ther Adv Neurol Disord 6:298–310
Gulani V, Calamante F, Shellock FG et al (2017) Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 16:564–570
Ognard J, Barrat JA, Cotton F et al (2021) A roadmap towards pollution prevention and sustainable development of Gadolinium. J Neuroradiol 48:409–411
Quattrocchi CC, Parillo M, Spani F et al (2023) Skin thickening of the scalp and high signal intensity of dentate nucleus in multiple sclerosis: association with linear versus macrocyclic gadolinium-based contrast agent administration. Invest Radiol 58:223–230. https://doi.org/10.1097/RLI.0000000000000929
Mallio CA, Rovira À, Parizel PM, Quattrocchi CC (2020) Exposure to gadolinium and neurotoxicity: current status of preclinical and clinical studies. Neuroradiology 62:925–934
Quattrocchi CC, Ramalho J, van der Molen AJ et al (2019) Standardized assessment of the signal intensity increase on unenhanced T1-weighted images in the brain: the European Gadolinium Retention Evaluation Consortium (GREC) Task Force position statement. Eur Radiol 29:3959–3967
Quattrocchi CC, van der Molen AJ (2017) Gadolinium retention in the body and brain: is it time for an international joint research effort? Radiology 282:12–16
Kanda T, Ishii K, Kawaguchi H et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173
Saade C, Bou-Fakhredin R, Yousem DM et al (2018) Gadolinium and multiple sclerosis: vessels, barriers of the brain, and glymphatics. AJNR Am J Neuroradiol 39:2168–2176
Minagar A, Alexander JS (2003) Blood-brain barrier disruption in multiple sclerosis. Mult Scler 9:540–549
Lassmann H (2019) Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. https://doi.org/10.3389/FIMMU.2018.03116
Barkhof F, Scheltens P, Frequin STFM et al (1992) Relapsing-remitting multiple sclerosis: sequential enhanced MR imaging vs clinical findings in determining disease activity. AJR Am J Roentgenol 159:1041–1047
Lassmann H (2008) The pathologic substrate of magnetic resonance alterations in multiple sclerosis. Neuroimaging Clin N Am 18:563–576
Koudriavtseva T, Thompson AJ, Fiorelli M et al (1997) Gadolinium enhanced MRI predicts clinical and MRI disease activity in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 62:285–287
Cotton F, Weiner HL, Jolesz FA, Guttmann CRG (2003) MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology 60:640–646
Burnham JA, Wright RR, Dreisbach J, Murray RS (1991) The effect of high-dose steroids on MRI gadolinium enhancement in acute demyelinating lesions. Neurology 41:1349–1349
Thompson AJ, Kermode AG, Wicks D et al (1991) Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol 29:53–62
Tremlett H, Zhao Y, Joseph J et al (2008) Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 79:1368–1374
Koch MW, Mostert J, Greenfield J et al (2020) Gadolinium enhancement on cranial MRI in multiple sclerosis is age dependent. J Neurol 267:2619–2624
Brownlee WJ, Altmann DR, Prados F et al (2019) Early imaging predictors of long-term outcomes in relapse-onset multiple sclerosis. Brain 142:2276–2287
Filippi M, Preziosa P, Banwell BL et al (2019) Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain 142:1858–1875
European Medicines Agency. EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans (21 July 2017). https://www.ema.europa.eu/en/documents/referral/gadolinium-article-31-referral-emas-final-opinion-confirms-restrictions-use-linear-gadolinium-agents_en-0.pdf. Accessed 20 Feb 2023
FDA Drug Safety Podcast: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings | FDA. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-drug-safety-podcast-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body-requires. Accessed 20 Feb 2023
Wijburg MT, Warnke C, McGuigan C et al (2021) Pharmacovigilance during treatment of multiple sclerosis: early recognition of CNS complications. J Neurol Neurosurg Psychiatry 92:177–188
Mallio CA, Quattrocchi CC, Rovira À, Parizel PM (2020) Gadolinium deposition safety: seeking the patient’s perspective. AJNR Am J Neuroradiol 41:944–946
Mallio CA, Piervincenzi C, Gianolio E et al (2019) Absence of dentate nucleus resting-state functional connectivity changes in nonneurological patients with gadolinium-related hyperintensity on T1 -weighted images. J Magn Reson Imaging 50:445–455
Mallio CA, Piervincenzi C, Carducci F et al (2020) Within-network brain connectivity in Crohn’s disease patients with gadolinium deposition in the cerebellum. Neuroradiology 62:833–841
Wiendl H, Gold R, Berger T et al (2021) Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord 14:17562864211039648
Fernandes L, Allen CM, Williams T et al (2021) The contemporary role of MRI in the monitoring and management of people with multiple sclerosis in the UK. Mult Scler Relat Disord 55:103190
Blumfield E, Swenson DW, Iyer RS, Stanescu AL (2019) Gadolinium-based contrast agents - review of recent literature on magnetic resonance imaging signal intensity changes and tissue deposits, with emphasis on pediatric patients. Pediatr Radiol 49:448–457
Towbin AJ, Zhang B, Dillman JR (2021) Evaluation of the effect of multiple administrations of gadopentetate dimeglumine or gadoterate meglumine on brain T1-weighted hyperintensity in pediatric patients. Pediatr Radiol 51:2568–2580
Noda SM, Oztek MA, Stanescu AL et al (2022) Gadolinium retention: should pediatric radiologists be concerned, and how to frame conversations with families. Pediatr Radiol 52:345–353
Oh KY, Roberts VHJ, Schabel MC et al (2015) Gadolinium chelate contrast material in pregnancy: fetal biodistribution in the nonhuman primate. Radiology 276:110–118
Puac P, Rodríguez A, Vallejo C et al (2017) Safety of contrast material use during pregnancy and lactation. Magn Reson Imaging Clin N Am 25:787–797
Winterstein AG, Thai TN, Nduaguba S et al (2022) Risk of fetal or neonatal death or neonatal intensive care unit admission associated with gadolinium magnetic resonance imaging exposure during pregnancy. Am J Obstet Gynecol 228:465.e1–465.e11
Ray JG, Vermeulen MJ, Bharatha A et al (2016) Association between MRI Exposure during pregnancy and fetal and childhood outcomes. JAMA 316:952–961
Chen MM, Coakley FV, Kaimal A, Laros RK (2008) Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet Gynecol 112:333–340
Mervak BM, Altun E, McGinty KA et al (2019) MRI in pregnancy: indications and practical considerations. J Magn Reson Imaging 49:621–631
Gatta G, Di Grezia G, Cuccurullo V et al (2021) MRI in pregnancy and precision medicine: a review from literature. https://doi.org/10.3390/JPM12010009
ACR (2023) Manual on contrast media, v2023. American College of Radiology, USA. https://www.acr.org/-/media/%20ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed 19 Jun 2023
ESUR (2018). ESUR guidelines on contrast agents, v10. https://www.esur.org/wp-content/uploads/2022/03/ESUR-Guidelines-10_0-Final-Version.pdf. Accessed 20 Feb 2023
ACOG (2017) Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol 130:e210–e216
Webb JA, Thomsen HS (2013) Gadolinium contrast media during pregnancy and lactation. Acta Radiol 54:599–600
Wang PI, Chong ST, Kielar AZ et al (2012) Imaging of pregnant and lactating patients: part 1, evidence-based review and recommendations. AJR Am J Roentgenol 198:778–784
Kubik-Huch RA, Gottstein-Aalame NM, Frenzel T et al (2000) Gadopentetate dimeglumine excretion into human breast milk during lactation. Radiology 216:555–558
Sundgren PC, Leander P (2011) Is administration of gadolinium-based contrast media to pregnant women and small children justified? J Magn Reson Imaging 34:750–757
Proença F, Guerreiro C, Sá G, Reimão S (2021) Neuroimaging safety during pregnancy and lactation: a review. Neuroradiology 63:837–845
Little JT, Bookwalter CA (2020) Magnetic resonance safety: pregnancy and lactation. Magn Reson Imaging Clin N Am 28:509–516
van der Molen AJ, Geenen RWF, Dekkers AI (2023) Guideline safe use of contrast media part 3, Radiological Society of The Netherlands (NVvR). https://radiologen.nl/sites/default/files/Kwaliteit/guideline_safe_use_of_contrast_media_part_3_final_8nov2022_eng.pdf. Accessed 26 Mar 2023
van Waesberghe JH, Castelijns JA, Roser W et al (1997) Single-dose gadolinium with magnetization transfer versus triple-dose gadolinium in the MR detection of multiple sclerosis lesions. AJNR Am J Neuroradiol 18:1279–1285
Rovira A, Auger C, Huerga E et al (2017) Cumulative dose of macrocyclic gadolinium-based contrast agent improves detection of enhancing lesions in patients with multiple sclerosis. AJNR Am J Neuroradiol 38:1486–1493
Giesel FL, Runge V, Kirchin M et al (2010) Three-dimensional multiphase time-resolved low-dose contrast-enhanced magnetic resonance angiography using TWIST on a 32-channel coil at 3 T. J Comput Assist Tomogr 34:678–683
Loevner LA, Kolumban B, Hutóczki G et al (2023) Efficacy and safety of gadopiclenol for contrast-enhanced MRI of the central nervous system: the PICTURE randomized clinical trial. Invest Radiol 58:307–313
Gong E, Pauly JM, Wintermark M, Zaharchuk G (2018) Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging 48:330–340
Filippi M, Yousry T, Rocca MA et al (1997) Sensitivity of delayed gadolinium-enhanced MRI in multiple sclerosis. Acta Neurol Scand 95:331–334
Absinta M, Vuolo L, Rao A et al (2015) Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology 85:18–28
Okar SV, Reich DS (2022) Routine gadolinium use for MRI follow-up of multiple sclerosis: point-the role of leptomeningeal enhancement. AJR Am J Roentgenol 219:24–25
Aymerich FX, Auger C, Alcaide-Leon P et al (2017) Comparison between gadolinium-enhanced 2D T1-weighted gradient-echo and spin-echo sequences in the detection of active multiple sclerosis lesions on 3.0T MRI. Eur Radiol 27:1361–1368
Bapst B, Amegnizin JL, Vignaud A et al (2020) Post-contrast 3D T1-weighted TSE MR sequences (SPACE, CUBE, VISTA/BRAINVIEW, isoFSE, 3D MVOX): technical aspects and clinical applications. J Neuroradiol 47:358–368
Hodel J, Outteryck O, Ryo E et al (2014) Accuracy of postcontrast 3D turbo spin-echo MR sequence for the detection of enhanced inflammatory lesions in patients with multiple sclerosis. AJNR Am J Neuroradiol 35:519–523. https://doi.org/10.3174/AJNR.A3795
Mugler JP, Bao S, Mulkern RV et al (2000) Optimized single-slab three-dimensional spin-echo MR imaging of the brain. Radiology 216:891–899
de Panafieu A, Lecler A, Goujon A et al (2023) Contrast-enhanced 3D spin echo T1-weighted sequence outperforms 3D gradient echo T1-weighted sequence for the detection of multiple sclerosis lesions on 3.0 T brain MRI. Invest Radiol 58:314–319
Di Perri C, Dwyer MG, Wack DS et al (2009) Signal abnormalities on 1.5 and 3 Tesla brain MRI in multiple sclerosis patients and healthy controls. A morphological and spatial quantitative comparison study. Neuroimage 47:1352–1362
Do Amaral LLF, Fragoso DC, da Rocha AJ (2019) Improving acute demyelinating lesion detection: which T1-weighted magnetic resonance acquisition is more sensitive to gadolinium enhancement? Arq Neuropsiquiatr 77:485–492
Bastianello S, Gasperini C, Paolillo A et al (1998) Sensitivity of enhanced MR in multiple sclerosis: effects of contrast dose and magnetization transfer contrast. AJNR Am J Neuroradiol 19:1863–1867
Algin O, Hakyemez B, Taşkapilioǧlu Ö et al (2010) Imaging of active multiple sclerosis plaques: efficiency of contrast-enhanced magnetization transfer subtraction technique. Diagn Interv Radiol 16:106–111
Al-Saeed O, Ismail M, Athyal R, Sheikh M (2011) Fat-saturated post gadolinium T1 imaging of the brain in multiple sclerosis. Acta Radiol 52:570–574
Balashov KE, Aung LL, Dhib-Jalbut S, Keller IA (2011) Acute multiple sclerosis lesion: conversion of restricted diffusion due to vasogenic edema. J Neuroimaging 21:202–204
Bugnicourt J-M, Garcia P-Y, Monet P et al (2010) Teaching NeuroImages: marked reduced apparent diffusion coefficient in acute multiple sclerosis lesion. Neurology 74:e87
Rosso C, Remy P, Creange A et al (2006) Diffusion-weighted MR imaging characteristics of an acute strokelike form of multiple sclerosis. AJNR Am J Neuroradiol 27:1006–1008
Rovira A, Pericot I, Alonso J et al (2002) Serial diffusion-weighted MR imaging and proton MR spectroscopy of acute large demyelinating brain lesions: case report. AJNR Am J Neuroradiol 23:989–994
Eisele P, Szabo K, Griebe M et al (2012) Reduced diffusion in a subset of acute MS lesions: a serial multiparametric MRI study. AJNR Am J Neuroradiol 33:1369–1373
Lucchinetti C, Brück W, Parisi J et al (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Rigby H, Maloney W, Bhan V (2012) Diagnostic considerations in acute MS lesions with restricted diffusion on MRI. Can J Neurol Sci 39:525–526
Tievsky AL, Ptak T, Farkas J (1999) Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am J Neuroradiol 20:1491–1499
Balashov KE, Lindzen E (2012) Acute demyelinating lesions with restricted diffusion in multiple sclerosis. Mult Scler 18:1745–1753
Gupta A, Al-Dasuqi K, Xia F et al (2017) The use of noncontrast quantitative MRI to detect gadolinium-enhancing multiple sclerosis brain lesions: a systematic review and meta-analysis. AJNR Am J Neuroradiol 38:1317–1322
Abdoli M, Chakraborty S, MacLean HJ, Freedman MS (2016) The evaluation of MRI diffusion values of active demyelinating lesions in multiple sclerosis. Mult Scler Relat Disord 10:97–102
Sacco S, Caverzasi E, Papinutto N et al (2020) Neurite orientation dispersion and density imaging for assessing acute inflammation and lesion evolution in MS. AJNR Am J Neuroradiol 41:2219–2226
Caruana G, Pessini LM, Cannella R et al (2020) Texture analysis in susceptibility-weighted imaging may be useful to differentiate acute from chronic multiple sclerosis lesions. Eur Radiol 30:6348–6356
Yu O, Mauss Y, Zollner G et al (1999) Distinct patterns of active and non-active plaques using texture analysis on brain NMR images in multiple sclerosis patients: preliminary results. Magn Reson Imaging 17:1261–1267
Michoux N, Guillet A, Rommel D et al (2015) Texture analysis of T2-weighted MR images to assess acute inflammation in brain MS lesions. PLoS One 10
Zhang Y, Gauthier SA, Gupta A et al (2016) Magnetic susceptibility from quantitative susceptibility mapping can differentiate new enhancing from nonenhancing multiple sclerosis lesions without gadolinium injection. AJNR Am J Neuroradiol 37:1794–1799
Caruana G, Auger C, Pessini LM et al (2022) SWI as an alternative to contrast-enhanced imaging to detect acute MS lesions. AJNR Am J Neuroradiol 43:534–539
Vinayagamani S, Sabarish S, Nair SS et al (2021) Quantitative susceptibility-weighted imaging in predicting disease activity in multiple sclerosis. Neuroradiology 63:1061–1069
Narayana PA, Coronado I, Sujit SJ et al (2020) Deep learning for predicting enhancing lesions in multiple sclerosis from noncontrast MRI. Radiology 294:398–404
Vargas WS, Monohan E, Pandya S et al (2015) Measuring longitudinal myelin water fraction in new multiple sclerosis lesions. NeuroImage Clin 9:369–375
Filippi M, Rocca MA, Martino G et al (1998) Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol 43:809–814
de la Peña MJ, Peña IC, García PG-P et al (2019) Early perfusion changes in multiple sclerosis patients as assessed by MRI using arterial spin labeling. Acta Radiol Open 8:2058460119894214
Acknowledgements
The ESMRMB-GREC (Gadolinium Research & Education Committee) is a group of multidisciplinary ESMRMB members, including clinicians, scientists, chemists, physicists, pathologists, and clinical epidemiologists who all share a common interest in the study of gadolinium-based contrast agents in a wide variety of clinical and preclinical conditions. The list of authors includes ESMRMB-GREC members (A. R., F. M. D.; A. J. M., C. A. M., C. A., C. Q.) and members of the Multiple Sclerosis Working Group of the European Society of Neuroradiology (ESNR) (A. R., L. H., J. H., M. S., M. P. W., B. J., T. Y.), who have written, revised and approved the final version of the manuscript
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Rovira.
Conflict of interest
(a) Àlex Rovira serves/ed on scientific advisory boards for Novartis, Sanofi-Genzyme, Icometrix, SyntheticMR, Bayer Healthcare, Biogen, and OLEA Medical, and has received speaker honoraria from Bayer Healthcare, Sanofi-Genzyme, Bracco Imaging, Merck-Serono, Teva Pharmaceutical Industries Ltd, Novartis, Roche, and Biogen.
(b) Fabio Doniselli, Cristina Auger, Jérôme Hodel, Mariasavina Severino, Bas Jasperse, Tarek Yousry, and Carlo Augusto Mallio do not have any conflict of interest to disclosure.
(c) Lukas Haider was supported by an ESNR (European Society of Neuro-Radiology) Research Fellowship and the ECTRIMS-MAGNIMS Research Fellowship and received funding from the Austrian MS Society.
(d) M. P. Wattjes received speaker or consultancy honoraria from Alexion, Bayer Healthcare, Biogen, Biologix, Celgene, Genilac, Imcyse, IXICO, Icometrix, Medison, Merck-Serono, Novartis, Roche, and Sanofi-Genzyme; publication royalties from Springer and Elsevier.
(e) A. J. van der Molen receives consultancy fees from Guerbet as a member of the Expert Group for Gadopiclenol in The Netherlands. He is a member of the ESUR-CMSC whose 2-yearly meetings have received support from Bayer Healthcare, Bracco Imaging, GE HealthCare, and Guerbet.
(f) Carlo Cosimo Quattrocchi has signed speaker contracts with Bayer Healthcare, Bracco Imaging, and Guerbet. He is co-chair of the ESMRMB-GREC Working Group whose yearly meetings have received unconditional support from Bayer Healthcare, Bracco Imaging, GE HealthCare, and Guerbet. He is a member of the ESUR-CMSC whose 2-yearly meetings have received support from Bayer Healthcare, Bracco Imaging, GE HealthCare, and Guerbet.
Statistics and biometry
The authors have significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because this is a review article.
Ethical approval
Institutional Review Board approval was not required because this is a review article.
Study subjects or cohort overlap
No study subject or cohort overlap reported.
Methodology
• Multicentre study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rovira, À., Doniselli, F.M., Auger, C. et al. Use of gadolinium-based contrast agents in multiple sclerosis: a review by the ESMRMB-GREC and ESNR Multiple Sclerosis Working Group. Eur Radiol 34, 1726–1735 (2024). https://doi.org/10.1007/s00330-023-10151-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10151-y