Abstract
Objectives
CT perfusion (CTP) has been suggested to increase the rate of large vessel occlusion (LVO) detection in patients suspected of acute ischemic stroke (AIS) if used in addition to a standard diagnostic imaging regime of CT angiography (CTA) and non-contrast CT (NCCT). The aim of this study was to estimate the costs and health effects of additional CTP for endovascular treatment (EVT)–eligible occlusion detection using model-based analyses.
Methods
In this Dutch, nationwide retrospective cohort study with model-based health economic evaluation, data from 701 EVT-treated patients with available CTP results were included (January 2018–March 2022; trialregister.nl:NL7974). We compared a cohort undergoing NCCT, CTA, and CTP (NCCT + CTA + CTP) with a generated counterfactual where NCCT and CTA (NCCT + CTA) was used for LVO detection. The NCCT + CTA strategy was simulated using diagnostic accuracy values and EVT effects from the literature. A Markov model was used to simulate 10-year follow-up. We adopted a healthcare payer perspective for costs in euros and health gains in quality-adjusted life years (QALYs). The primary outcome was the net monetary benefit (NMB) at a willingness to pay of €80,000; secondary outcomes were the difference between LVO detection strategies in QALYs (ΔQALY) and costs (ΔCosts) per LVO patient.
Results
We included 701 patients (median age: 72, IQR: [62–81]) years). Per LVO patient, CTP-based occlusion detection resulted in cost savings (ΔCosts median: € − 2671, IQR: [€ − 4721; € − 731]), a health gain (ΔQALY median: 0.073, IQR: [0.044; 0.104]), and a positive NMB (median: €8436, IQR: [5565; 11,876]) per LVO patient.
Conclusion
CTP-based screening of suspected stroke patients for an endovascular treatment eligible large vessel occlusion was cost-effective.
Clinical relevance statement.
Although CTP-based patient selection for endovascular treatment has been recently suggested to result in worse patient outcomes after ischemic stroke, an alternative CTP-based screening for endovascular treatable occlusions is cost-effective.
Key Points
• Using CT perfusion to detect an endovascular treatment-eligible occlusions resulted in a health gain and cost savings during 10 years of follow-up.
• Depending on the screening costs related to the number of patients needed to image with CT perfusion, cost savings could be considerable (median: € − 3857, IQR: [€ − 5907; € − 1916] per patient).
• As the gain in quality adjusted life years was most affected by the sensitivity of CT perfusion-based occlusion detection, additional studies for the diagnostic accuracy of CT perfusion for occlusion detection are required.
Similar content being viewed by others
Introduction
Brain tissue perfusion maps derived from computed tomography perfusion (CTP) have been suggested to improve occlusion detection in acute ischemic stroke (AIS) patients if used in addition to CT angiography (CTA) and non-contrast CT (NCCT) [1,2,3,4]. Although CTP is primarily considered to select patients for endovascular treatment (EVT) [5], screening all suspected AIS patients presenting within 6 h after symptom onset with CTP could also enhance the detection of patients with a large vessel occlusion (LVO) EVT, resulting in more patients who benefit from EVT and less missed occlusions [6]. Although EVT compared to best medical management is considered cost-effective [7], it remains unclear to what extent the direct costs of screening a large group of patients with CTP for EVT-eligible occlusions result in long-term health gains and cost savings.
Several studies found that adding CTP to an imaging regime of non-contrast-enhanced CT (NCCT) and CT angiography (CTA) enhances the sensitivity for arterial occlusion detection [1,2,3,4]. Moreover, the sensitivity gain of adding CTP was between 0 and 20% [1,2,3,4] — depending on the experience of the neuroradiologist and the occlusion location. Since EVT has vastly improved outcomes of AIS patients with a large vessel occlusion (LVO) [6, 8], the total quality-adjusted life-years (QALYs) of patients after an AIS can be increased by providing EVT to all eligible patients.
Two previous health economic evaluations concluded that CTP was cost-effective when used jointly for EVT- and intravenous thrombolysis–eligible occlusion detection and to exclude patients with severe ischemia for whom EVT may potentially be harmful [9, 10]. However, these studies considered deterministic fixed estimates for the value of additional CTP for EVT-eligible LVO detection that do not correspond with recent findings [1, 2]. In addition, the benefit of CTP-based occlusion detection may be higher for less-experienced physicians compared to experienced neuroradiologists. Furthermore, variations in the proportion of patients with an EVT-eligible occlusion compared to the overall population presenting with AIS symptoms at the emergency department alter the number of patients needed to image (NNI) to detect an LVO and increase the total costs of CTP. Finally, the two studies considered a US perspective that might not apply to other healthcare systems [9, 10].
In this study, we aimed to estimate the long-term costs and health effects of adding CTP (NCCT + CTA + CTP) for LVO detection to a standard imaging protocol of NCCT and CTA (NCCT + CTA) in Dutch patients suspected of AIS presenting within 6 h after symptom onset at an EVT-capable hospital. Furthermore, we aimed to analyze the effect of variations in the sensitivity of NCCT + CTA + CTP compared to NCCT + CTA-based LVO detection, the number of CTPs needed to acquire (NNI) before an LVO was detected, and the benefit of EVT.
Methods
Study design
In this study, a cohort of patients that received EVT after NCCT + CTA + CTP-based LVO detection with 90-day functional outcome according to the modified Rankin Scale (mRS) was used to simulate long-term mRS and a cohort with 90-day mRS after NCCT + CTA-based LVO detection. An EVT-eligible LVO was defined as occlusion of the internal carotid artery-(terminus) (ICA/ICA-T), the M1 or proximal M2 segment of the middle cerebral artery. To generate the NCCT + CTA cohort, a percentage of patients was simulated as if they did not receive EVT due to a missed LVO. This percentage of missed LVOs was based on the difference in sensitivity between NCCT + CTA + CTP- and NCCT + CTA-based LVO detection found in a literature search (Online Supplement A); this was referred to as the sensitivity difference [1,2,3,4]. We did not consider the difference in specificity or positive predictive value due to additional CTP because the relative difference between the imaging strategies would be small and the negative health effects and additional costs of a futile transfer to the angio suite are assumed to be negligible on a population basis. For patients that would not have received EVT under the NCCT + CTA regime, the observed mRS in the included cohort was reduced using available ORs for EVT effect from the literature (Online Supplement B) [6, 11]. To include the additional costs of CTP in the NCCT + CTA + CTP arm, the NNI was used to add additional screening costs per patient with an LVO; for each detected LVO, there would be numerous patients without an LVO that were screened with CTP and related costs (Online Supplement C).
Patient level data
We included patients from the Cost-effectiveness of CT perfusion for Patients with Acute Ischemic Stroke (CLEOPATRA) healthcare evaluation study [12] in the Netherlands that recruited patients originally included in the MR CLEAN NO IV [13], MR CLEAN MED [14], and MR CLEAN Registry [15] (January 2018–March 2022; trialregister.nl:NL7974). We only included data from EVT-treated patients with available CTP imaging who presented within 6 h after stroke symptom onset in an EVT capable hospital. Minor protocol deviations and CTP processing methods are available in Online Supplement D and E. This study was conducted according to the Helsinki agreement; part of the data has previously been reported (Online Supplement F).
Modeling approach
We simulated 5- and 10-year follow-up using a Markov model with patient-level microsimulations. The Markov model was previously described and consisted of a short-term 90-day post-AIS model followed by a long-term yearly model to simulate functional outcome using the modified Rankin Scale (mRS) (Fig. 1) [12, 16]. In the short-term model, we simulated the 90-day mRS of patients that received EVT and those who did not based on NCCT + CTA + CTP- or NCCT + CTA-based LVO detection. In the long-term model, we simulated yearly mRS deterioration after 90 days based on the probability of stroke recurrence [17] and death [18] inflated with patient-specific hazard ratios (HR) [19]. Python scripts for the simulations are made publicly available (github.com/henkvanvoorst92/CLEOPATRA).
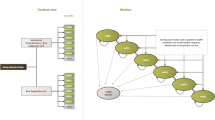
Markov model structure. A Patients presenting within 6 h after stroke symptom onset at an endovascular treatment (EVT)–capable stroke center are subject to one of the following diagnostic imaging protocols for EVT-eligible occlusion detection: (1) non-contrast CT (NCCT), CT angiography (CTA), and CT perfusion (CTP), or (2) NCCT and CTA. No EVT*: In the NCCT + CTA + CTP arm, the number of patients without an EVT-eligible occlusion (no EVT) was computed using the number needed to image (NNI) calculations. Costs of CTP-based screening of non-EVT-eligible occlusions were multiplied with the NNI and added to the overall costs of this simulated arm, in the models’ CTP—no EVT group did not suffer any health consequences and was not further simulated. In the NCCT + CTA arm, the no EVT compromised all patients from the NCCT + CTA + CTP arm in addition to all patients that were missed due to less optimal EVT-eligible occlusion screening. The long-term modified Rankin Scale (mRS) of the missed EVT-eligible occlusion group was further simulated. ** The sensitivity gain due to CTP-based EVT-eligible occlusion detection was used to compute the size of the group of missed EVT-eligible occlusions if a diagnostic imaging protocol consisting of NCCT + CTA was used. B The 90-day mRS was modeled after EVT or no EVT. C Yearly mRS transitions were modeled based on death and recurrent stroke rates beyond 90 days after stroke. EVT, endovascular treatment; NCCT, non-contrast enhanced CT; CTA, CT angiography; mRS, modified Rankin scale
Costs and QALYs
We used mRS over the simulated period to compute cumulative costs from a healthcare payer perspective and QALYs [20]. The methodology for acute care and mRS related follow-up QALYs and costs has previously been described [12, 20]. QALYs were computed per mRS sub-score per year based on 391 patients with 2-year follow-up and available EuroQoL 5D questionnaires [8]. Follow-up costs for the first, second, and third year onward per mRS sub-score included the following: acute setting treatment cost, in-hospital costs, outpatient clinic visits, rehabilitation, formal homecare, and long-term institutionalized costs [8]. Acute care costs included NCCT, CTA, CTP, EVT, and IVT if applicable based on reference prices from the institute of Medical Technological Assessment, Rotterdam, the Netherlands [12, 20]. Acute care costs were increased by 42% to account for hospital overhead costs according to Dutch cost-pricing standards [21]. We pre-defined costs per CTP of €251.40 based on the following assumptions and data: €129 for the CTP acquisition [21], costs for acute care personnel (€94) [21], €20 for the CTP software license based on expert opinion. EVT costs were €9924.50 consisting of material costs and 1.5 h of personnel costs for (neuro-) interventionist, 1 anesthesiologist, 2 radiology assistants, and 2 anesthesia assistants [12, 20]. IVT costs of €950.82 were extracted from medicijnkosten.nl. Simulations started in 2022. An annual discounting rate of 4% for QALYs and 1.5% for costs was used to compute present values [22]. Inflation-based cost adjustments were made using historical and forecasted inflation rates [23, 24].
Outcome measures
Net monetary benefit (NMB: Formula 1) at a willingness to pay (WTP) of €80,000 per QALY was the primary outcome. Secondary outcomes were the differences in cost (ΔCosts) and quality-adjusted life-years (ΔQALYs) between the intervention (NCCT + CTA + CTP-based LVO detection) and control (NCCT + CTA-based LVO detection) arm. All results were reported as cumulative values over the simulated period with median and interquartile range (IQR) per simulated patient with an LVO.
Baseline and sensitivity analyses
Mean values of the input parameters were used for the baseline simulation considering a 5-year follow-up period. A 10% increase and decrease of all input parameters were used to simulate one-way sensitivity results to assess the outcome variability due to input parameter changes. Probabilistic sensitivity analyses (PSAs) were performed to represent input parameter uncertainty in the outcome measures. For the PSAs, 1000 cohorts were sampled with replacement from the original data. All model input parameters and distributions are described in Table 1.
Dedicated PSAs
We performed dedicated PSAs to analyze the effect of major factors that affect the cost-effectiveness of CTP-based EVT-eligible occlusion detection.
-
A)
To summarize the variations in sensitivity difference between NCCT + CTA + CTP- and NCCT + CTA-based LVO detection, we performed a systematic search on PubMed combining terms related to CTP, CTA, sensitivity, and stroke with an AND term (Supplement A). We defined ranges of sensitivity difference for ICA, M1, and M2 occlusions separately based on the literature [1,2,3,4] (Table 2). For ICA occlusions, we used a baseline sensitivity difference of 8% and varied values with increments of 2% between 4 and 12% [4]. For M1 and M2 occlusions, we used a baseline sensitivity difference of 16% and varied values with increments of 2% between 12 and 20% [2, 4].
-
B)
We performed a sensitivity analysis for the treatment effect of EVT that was used to generate the 90-day mRS of patients that would not receive EVT in the NCCT + CTA arm (Online Supplement B). The OR for the treatment effect of EVT (OR: 1.67; 95%CI: [1.21; 2.30]) from the MR CLEAN trial [11], the trial with the most conservative EVT benefit [6], was altered with − 0.3, + 0.3, and + 0.82 [6].
-
C)
We used the number needed to image (NNI) to accrue for the costs of all CTPs made, including CTP costs for patients not receiving EVT. We varied the NNI between 4.3 and 8.3, based on previously reported values in the literature and ambulance data from two urban regions in the Netherlands (detailed computations in Supplement C). We assumed that 50–60% of all suspected stroke patients admitted to an EVT-capable hospital have an AIS [25] (Table 3). Of all AIS patients, 24–46% have an LVO [26].
Results
Descriptive statistics
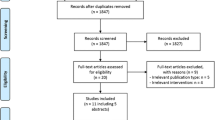
We included 701 (390/701 male, median age 72 [IQR: 62; 81]) of 1122 patients available in the CLEOPATRA database for the simulations. An inclusion flow chart is available in Fig. 2. Patients were excluded due to an onset of stroke symptoms to groin puncture time beyond 6 h (n = 172), absence of CTP source data (n = 91), no accurate CTP results after processing (n = 86), double inclusion (n = 65), and an unknown occlusion location or a posterior circulation occlusion (n = 7). Baseline characteristics for the total population and subgroups based on occlusion location are presented in Table 4.
Flowchart of patient selection. *CTP source data without time information or CTP source data not available due to local storage in the primary stroke center. †Reasons for inaccurate CTP results include severe patient motion, severe curve truncation, no timely contrast arrival or incorrect timing CTP, or severe artifacts in CTP source data. CTP, CT perfusion; ICA, internal carotid artery
Baseline model and one-way sensitivity
Using the mean input values, an NNI of 8.3, and 5 years of follow-up for the baseline simulations resulted in a gain of health (ΔQALY: 0.049) and higher costs (ΔCosts: €482), with a positive NMB (€3447) when NCCT + CTA + CTP would be used compared to NCCT + CTA for LVO detection. Figure 3 describes the ten most influential model parameters in a one-way sensitivity analysis. The amount of QALYs attributed to mRS 0–3 were the most important factors affecting the NMB. Costs of CTP and EVT were the most influential cost factors affecting the NMB; long-term follow-up care costs only had a limited effect on the NMB. Figure S3 (Online Supplement G) contains a Kaplan-Maier plot describing the simulated 10-year survival.
Tornado diagram of the one-way sensitivity analyses. Changes in average NMB compared to the baseline (€3447) are depicted for a 10% increase (black) and decrease (gray) of the ten most influential model input variables. A 5-year horizon was used with an NNI of 8.3 and a baseline sensitivity gain. Variations in NNI, sensitivity gain, and EVT effect were not considered for this analysis. EVT, endovascular treatment; QALY, quality-adjusted life-years; mRS, modified Rankin Scale; CTP, CT perfusion; NMB, net monetary benefit
Probabilistic sensitivity analysis
The incremental cost-effectiveness ratio plots per occlusion location and for all occlusion locations together are visualized in Fig. 4. Considering an NNI of 8.3, the baseline sensitivity difference, and a follow-up horizon of 5 years resulted per EVT-eligible LVO patient in an increase in costs (ΔCosts median: €777, IQR: [€ − 290; 1825]), a health gain (ΔQALY median: 0.048, IQR: [0.032; 0.064]), and a positive NMB (median: €3015, IQR: [€1455; €4779) when NCCT + CTA + CTP would be used compared to NCCT + CTA for LVO detection. Using similar settings but a 10-year follow-up horizon resulted in a cost-saving (ΔCosts median: € − 2671, IQR: [€ − 4721; € − 731]), a health gain (ΔQALY median: 0.073, IQR: [0.044; 0.104]), and a positive NMB (median: €8436, IQR: [€5565; €11,876]).
Incremental cost-effectiveness ratio (ICER) plot per occlusion location. Incremental cost-effectiveness ratio plots are presented for (A) ICA, (B) M1, (C) M2, and (D) all simulated patients together. Simulations considered the baseline sensitivity gain per occlusion location, and a 5-year follow-up period. Panels A–C do not include the CTP screening costs; panel D does include the CTP screening costs using the NNI multiplier (NNI = 8.3). Positive values represent more costs or QALYs when CTP is included in an imaging protocol consisting of NCCT and CTA for occlusion detection. The dashed diagonal line represents the willingness to pay of €80,000 per QALY
Dedicated sensitivity analyses
Variations in NMB due to the follow-up horizon, NNI, and sensitivity difference are graphically presented in Fig. 5. Table 5 contain the results for ΔQALY, ΔCosts, NMB, and the fraction of the simulations that were cost-effective (below the WTP line; an NMB > 0) at a WTP of €80,000 for varying scenarios. More extensive results for varying model parameters are described in Online Supplement G.
Net monetary benefit for dedicated probabilistic sensitivity analyses. Each panel shows the NMB at a willingness to pay of €80,000 per quality-adjusted life years (QALY) on the y-axis. A positive net monetary benefit implies that the additional costs of CTP-based screening, EVT, and long-term care costs are lower than the health gain. On the x-axis, the percentage point in sensitivity difference relative to the baseline values of additional CTP (NCCT + CTA + CTP) compared to NCCT + CTA is depicted. The baseline sensitivity difference was 6% for ICA occlusions and 16% for M1 and M2 occlusions. The colors represent the median OR for the treatment effect used for simulations. Panels A–D depict varying NNI and years of follow-up. (A) NNI of 4.3 considering 5-year follow-up. (B) NNI of 8.3 considering 5-year follow-up. (C) NNI of 4.3 considering 10-year follow-up. (D) NNI of 8.3 considering 10-year follow-up. NNI, number of patients needed to image; OR, odds ratio; WTP, willingness to pay; QALY, quality-adjusted life years; NMB, net monetary benefit
Between the upper and lower NNI bound additional costs or savings differed (ΔCosts 10-year follow-up: NNI = 4.3; median: € − 3857, IQR: [€ − 5907; € − 1916] vs. NNI = 8.3; median: € − 2671, IQR: [€ − 4721; € − 731]) while the QALYs were the same (ΔQALY median: 0.073, IQR: [0.044; 0.104]). Variations in sensitivity difference resulted in different health gains (ΔQALYs 10-year follow-up: sensitivity difference = baseline median: 0.073, IQR: [0.044; 0.104] vs. sensitivity difference = (baseline − 4%) median: 0.052, IQR: [0.031; 0.075] vs. sensitivity difference = (baseline + 4%) median: 0.094, IQR: [0.057–0.134]). Furthermore, variations in EVT-effect relative to the baseline effect found in the MR CLEAN trial (baseline OR: 1.67; 95%CI: [1.21; 2.30]) resulted in a limited difference in cost savings (NNI = 8.3 10-year follow-up: ΔCosts EVT-effect baseline median: € − 2671, IQR: [€ − 4721; € − 731]; baseline − 0.3 median: € − 2683, IQR: [€ − 4715; € − 836]; baseline + 0.3 median: € − 2592, IQR: [€ − 4622; € − 657]) and more profound variations in health gains (10-year follow-up: ΔQALYs baseline median: 0.073, IQR: [0.044; 0.104]; baseline − 0.3 median: 0.062, IQR: [0.034; 0.094]; baseline + 0.3: 0.082, IQR [0.052; 0.113]). NCCT + CTA + CTP-based LVO detection would not be cost-effective if we considered 5-year follow-up, an NNI of 8.3, and a sensitivity gain 8% below the baseline (ΔCosts median: €1872, IQR: [€1394; €2337]; ΔQALYs median: 0.021, IQR: [0.014; 0.028]; NMB median: € − 249, IQR: [€ − 925; €566]).
Discussion
In this cohort study with model-based health economic evaluation, we found that adding CTP to an imaging regime of CTA and NCCT for EVT-eligible large vessel occlusion detection followed by endovascular treatment, resulted in a cost savings (ΔCosts median: € − 2671, IQR: [€ − 4721; € − 731]), a health gain (ΔQALY median: 0.073, IQR: [0.044; 0.104]), and a positive net monetary benefit (median: €8436, IQR: [5565; 11,876]) during a 10-year follow-up horizon considering a healthcare payer perspective per patient with an LVO. Costs and health effects per CTP screened patients were small but unevenly distributed as only the group of patients with a missed occlusion would benefit. Around 2350 patients receive EVT per year in the Netherlands [27], for each year that CTP would be used to detect LVOs eligible for EVT 172 QALYs (IQR: [103; 244]) and €9.1 million (IQR: €13.9mln; €4.5mln) could be saved.
Similar to previous research, the use of CTP was found cost-effective [9, 10]. However, this study provides a more detailed description of factors causing changes in costs and health effects. To interpret these results, we need to consider the currently available evidence. First, NNI estimates might differ; a higher ischemic stroke prevalence or less CTPs with uninterpretable results would result in a lower NNI and thus result less CTPs per detected LVO and lower costs. Second, the benefit in LVO detection sensitivity of additional CTP to a diagnostic workup consisting of NCCT and CTA for AIS screening from previous reader studies varied depending on the physician’s experience [1,2,3,4]. The value of CTP-based screening might be higher for physicians with less experience in neuroimaging assessments. In this study we reported a wide variety of sensitivity differences as the current evidence comparing NCCT + CTA + CTP- with NCCT + CTA-based LVO detection is limited by a small sample size and suboptimal research designs [1,2,3,4]. Third, we used conservative ORs for the EVT treatment effect based on the MR CLEAN trial [12]. In recent years, EVT workflows have improved, improving the treatment effect of EVT [28]. By underestimating the effect of EVT, we might allow for better generalizability of our findings to settings with different stroke populations or physician’s experience with EVT, such as primary stroke centers or stroke centers in developing countries. Namely, due to the transfer of patients, the onset to groin time increases, negatively affecting the EVT effect [29]. Fourth, the variety of occlusion subtypes in our population might be different from the occlusion subtype distribution in other populations [6]. Fifth, costs and health effects due to negative side effects related to CTP were not considered. Allergic reactions and renal insufficiency due to a higher dose of intravenously administered contrast medium may be harmful [30, 31]. Furthermore, additional radiation exposure due to CTP might cause harm when considering long-term follow-up [32]. Since these side effects occur in a very small portion of the population, often have limited long-term effects, or occur in the far future, we expect this to only have a minor effect on the costs and health effects in this study. Sixth, current trials studying EVT for more distal occlusions might result in even higher health gains due to CTP-based occlusion detection [33, 34].
Our study has limitations. This is a model-based study using an observational cohort of only patients receiving EVT in the Netherlands, our findings might deviate in prospectively based trial cost-effectiveness analyses and in other patient populations. Specifically, we did not have data on long-term functional outcome follow-up, micro-level-estimated costs, nor did we include patients that did not receive EVT due to a missed LVO. Furthermore, we used cost data from a healthcare payer perspective neglecting indirect costs. Indirect costs related to poor patient outcomes such as a reduction in labor participation and higher out-of-pocket healthcare expenses might result in an even higher benefit of CTP-based EVT-eligible LVO screening.
Future research should analyze alternative diagnostic imaging strategies compared to the direct use of NCCT, CTA, and CTP used in this study. CTP could be considered after an inconclusive CTA and NCCT examination or in combination with patient characteristics. Although this study focused on radiologist-based CTP examination, recently developed artificial intelligence–based software might also improve occlusion detection and could therefore be cost-effective. Comparative studies are required to define the optimal occlusion detection strategy. Since we only considered EVT-eligible LVOs, future studies should consider the value of CTP for detecting EVT-eligible distal vessel occlusions or posterior circulation occlusions — which are generally more difficult to detect. Finally, future work should aim to determine stroke subtype statistics to compute the NNI and assess determinants for varying sensitivity differences due to CTP in a large reader study.
Conclusion
In this model-based health economic evaluation study, detecting more EVT eligible LVOs using CTP-based screening for patients presenting within 6 h after symptom onset was cost-effective due to a health gain and limited additional costs to a cost saving over 10-year follow-up. Health gains and cost savings might be more favorable for a lower number needed to image, a longer follow-up horizon, and a higher sensitivity difference of additional CTP compared to NCCT and CTA based large vessel occlusion detection.
Abbreviations
- AIS:
-
Acute ischemic stroke
- CLEOPATRA:
-
Cost-effectiveness of CT perfusion for Patients with Acute Ischemic Stroke
- CTA:
-
CT angiography
- CTP :
-
CT perfusion
- EVT :
-
Endovascular treatment
- ICA:
-
Internal carotid artery
- ICA-T:
-
Terminus of ICA
- ICER:
-
Incremental cost-effectiveness ratio
- IQR:
-
Interquartile range
- LVO:
-
Large vessel occlusion
- mRS:
-
Modified Rankin Scale
- NCCT:
-
Non-contrast CT
- NMB:
-
Net monetary benefit
- NNI:
-
Number needed to image
- PSA :
-
Probabilistic sensitivity analysis
- QALY:
-
Quality-adjusted life year
- ΔCosts:
-
Differences in cost
- ΔQALYs:
-
Differences in quality-adjusted life years
References
Becks MJ, Manniesing R, Vister J et al (2019) Brain CT perfusion improves intracranial vessel occlusion detection on CT angiography. J Neuroradiol 46(2):124–129
Bathla G, PillenahalliMaheshwarappa R, Soni N et al (2022) Do CT perfusion maps increase accuracy for detection of M2-MCA occlusions in acute ischemic stroke? J Stroke Cerebrovasc Dis 31(6):1–7
Hopyan J, Ciarallo A, Dowlatshahi D et al (2010) Certainty of stroke diagnosis: incremental benefit with CT perfusion over noncontrast CT and CT angiography. Radiology 255(1):142–153
Olive-Gadea M, Requena M, Diaz F et al (2021) Systematic CT perfusion acquisition in acute stroke increases vascular occlusion detection and thrombectomy rates. J Neurointerv Surg 14(12):1270–1273
Powers WJ, Rabinstein AA, Ackerson T et al (2018) Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49:46–110
Goyal M, Menon BK, van Zwam WH et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387(10029):1723–1731
Ospel JM, Kunz WG, McDonough RV et al (2023) Cost-effectiveness of endovascular treatment in large vessel occlusion stroke with mild prestroke disability: results from the HERMES Collaboration. Stroke 54(1):226–233
Van Den Berg LA, Berkhemer OA, Fransen PSS et al (2022) Economic evaluation of endovascular treatment for acute ischemic stroke. Stroke 29(2):968–975
Boltyenkov AT, Martinez G, Pandya A et al (2021) Cost-consequence analysis of advanced imaging in acute ischemic stroke care. Front Neurol 12(November):1–9
Martinez G, Katz JM, Pandya A et al (2021) Cost-effectiveness study of initial imaging selection in acute ischemic stroke care. J Am Coll Radiol 18(6):820–833
Berkhemer OA, Fransen PSS, Beumer D et al (2015) A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372(1):11–20
Koopman MS, Hoving JW, van Voorst H et al (2022) Cost-effectiveness of CT perfusion for patients with acute ischemic stroke (CLEOPATRA)-Study protocol for a healthcare evaluation study. Eur Stroke J
LeCouffe NE, Kappelhof M, Treurniet KM et al (2021) A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med 385(20):1833–1844
van der Steen W, van der Sluijs PM, van de Graaf RA et al (2022) Safety and efficacy of periprocedural antithrombotics in patients with successful reperfusion after endovascular stroke treatment. J Stroke Cerebrovasc Dis 31(10):106726
Jansen IGH, Mulder MJHL, Goldhoorn RJB (2018) Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ 360:
Van Voorst H, Kunz WG, Van Den Berg LA et al (2020) Quantified health and cost effects of faster endovascular treatment for large vessel ischemic stroke patients in the Netherlands. J Neurointerv Surg 1–8
Pennlert J, Eriksson M, Carlberg B, Wiklund PG (2014) Long-term risk and predictors of recurrent stroke beyond the acute phase. Stroke 45(6):1839–1841
Genootschap KA, Prognosetafel AG (2018). www ag-ai nl [Internet]. [accessed 2020 Sep 1]. 2018; Available from: https://www.ag-ai.nl/view.php?action=view&Pagina_Id=888
Hong KS, Saver JL (2010) Years of disability-adjusted life gained as a result of thrombolytic therapy for acute ischemic stroke. Stroke 41(3):471–477
Van Voorst H, Kunz WG, Van Den Berg LA et al (2021) Quantified health and cost effects of faster endovascular treatment for large vessel ischemic stroke patients in the Netherlands. J Neurointerv Surg 13(12):1099–1105
Roijen LH, Van der Linden N (2016). Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg
Hakkaart-van Roijen L, van der Linden N, Bouwmans C, Kanters T, Swan Tan S (2016) Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Dutch Natl Health Care Inst
(2020) Dutch Central Bureau of Statistics. Consumer price index [Internet]. [cited 2020 Sep 1]. Available from: opendata.cbs.nl/statline
Zorguitgaven | Volksgezondheid Toekomst Verkenning [Internet]. [cited 2020 Apr 1]. Available from: https://www.vtv2018.nl/zorguitgaven
Duvekot MHC, Venema E, Rozeman AD et al (2021) Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): a prospective observational study. Lancet Neurol 20(3):213–221
Rennert RC, Wali AR, Steinberg JA et al (2019) Epidemiology, natural history, and clinical presentation of large vessel ischemic stroke. Clin Neurosurg 1(85):S4-8
(2021) Jaarcijfers acuut herseninfarct [Internet]. Hart en Vaat cijfers. [accessed 2023 May 16]. Available from: https://www.hartenvaatcijfers.nl/jaarcijfers/jaarcijfers-acuut-herseninfarct-f323d
Janssen PM, Venema E, Di Dippel WJ (2019) Effect of workflow improvements in endovascular stroke treatment: a systematic review and meta-analysis. Stroke 50(3):665–674
Saver JL, Goyal M, Van Der Lugt A et al (2016) Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: ameta-analysis. JAMA 316(12):1279–1288
Hopyan JJ, Gladstone DJ, Mallia G et al (2008) Renal safety of CT angiography and perfusion imaging in the emergency evaluation of acute stroke. AJNR Am J Neuroradiol 29(10):1826–1830
Mortelé KJ, Oliva MR, Ondategui S, Ros PR, Silverman SG (2005) Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol 184(1):31–34
Sowby FD (1981) Annals of the ICRP. Ann ICRP 6(1):1
(2022) EndovaSCular TreAtment to imProve outcomEs for Medium Vessel Occlusions (ESCAPE-MeVO Trial) - Full Text View - ClinicalTrials.gov [Internet]. [accessed 2022 Jul 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT05151172
(2022) DISTAL: A landmark study looking to elucidate endovascular treatment outcomes in MeVO stroke [Internet]. [accessed 2022 Jul 22]. Available from: https://neuronewsinternational.com/distal-study-evt-mevo-stroke/
Acknowledgements
For the CONTRAST consortium:
Research leaders
Diederik Dippel (MD, PhD),1 Charles Majoie (MD, PhD).3
Consortium coordinator:
Rick van Nuland, (PhD).17
Imaging assessment committee
Charles Majoie (MD, PhD) – Chair,3 Aad van der Lugt (MD, PhD) – Chair,1 Adriaan van Es, (MD, PhD),1,2 Pieter-Jan van Doormaal (MD),1 René van den Berg, (MD, PhD),3 Ludo Beenen (MD),3 Bart Emmer (MD, PhD),3 Stefan Roosendaal (MD, PhD),3 Wim van Zwam (MD, PhD),4 Alida Annechien Postma (MD, PhD),18 Lonneke Yo (MD, PhD),6 Menno Krietemeijer (MD),6 Geert Lycklama (MD, PhD),7 Jasper Martens (MD),8 Sebastiaan Hammer (MD, PhD),10 Anton Meijer (MD, PhD),10 Reinoud Bokkers (MD, PhD),15 Anouk van der Hoorn (MD, PhD),15 Ido van den Wijngaard (MD, PhD),2,7 Albert Yoo (MD, PhD),19 Dick Gerrits (MD).20
Adverse event committee
Robert van Oostenbrugge (MD, PhD) – Chair,4 Bart Emmer (MD, PhD),3 Jonathan M. Coutinho (MD, PhD),3 Martine Truijman (MD, PhD),4 Julie Staals (MD, PHD),4 Bart van der Worp (MD, PhD),5 J. Boogaarts (MD, PhD),10 Ben Jansen (MD, PhD),16 Sanne Zinkstok (MD, PhD).21
Outcome assessment committee
Yvo Roos (MD, PhD) – Chair,3 Peter Koudstaal (MD, PhD),1 Diederik Dippel (MD, PhD),1 Jonathan M. Coutinho (MD, PhD),3 Koos Keizer (MD, PhD),5 Sanne Manschot (MD, PhD),7 Jelis Boiten (MD, PhD),7 Henk Kerkhoff (MD, PhD),14 Ido van den Wijngaard (MD, PhD)2,7
Data management group
Hester Lingsma (PhD),1 Diederik Dippel (MD, PhD),1 Vicky Chalos (MD),1 Olvert Berkhemer (MD, PhD)1,3
Imaging data management
Aad van der Lugt (MD, PhD),1 Charles Majoie (MD, PhD),3 Adriaan Versteeg,1 Lennard Wolff (MD),1 Matthijs van der Sluijs (MD),1 Henk van Voorst (MD),3 Manon Tolhuisen (MSc),3
Biomaterials and translational group
Hugo ten Cate (MD, PhD),4 Moniek de Maat (PhD),1 Samantha Donse-Donkel (MD),1 Heleen van Beusekom (PhD),1 Aladdin Taha (MD),1 Aarazo Barakzie (MD).1
Local collaborators
Vicky Chalos (MD, PhD),1 Rob van de Graaf (MD, PhD),1 Wouter van der Steen (MD),1 Aladdin Taha (MD),1 Samantha Donse-Donkel (MD),1 Lennard Wolff (MD),1 Kilian Treurniet (MD),3 Sophie van den Berg (MD),3 Natalie LeCouffe (MD),3 Manon Kappelhof (MD),3 Rik Reinink (MD),3 Manon Tolhuisen (MD),3 Leon Rinkel (MD),3 Josje Brouwer (MD),3 Agnetha Bruggeman (MD),3 Henk van Voorst (MD),3 Robert-Jan Goldhoorn (MD),4 Wouter Hinsenveld (MD),4 Anne Pirson (MD),4 Susan Olthuis (MD),4 Simone Uniken Venema (MD),5 Sjan Teeselink (MD),10 Lotte Sondag (MD),10 Sabine Collette (MD).15
Research nurses
Martin Sterrenberg,1 Naziha El Ghannouti,1 Laurine van der Steen,3 Sabrina Verheesen,4 Jeannique Vranken,4 Ayla van Ahee,5 Hester Bongenaar,6 Maylee Smallegange,6 Lida Tilet,6 Joke de Meris,7 Michelle Simons,8 Wilma Pellikaan,9 Wilma van Wijngaarden,9 Kitty Blauwendraat,9 Yvonne Drabbe,11 Michelle Sandiman-Lefeber,11 Anke Katthöfer,11 Eva Ponjee,12 Rieke Eilander,12 Anja van Loon,13 Karin Kraus,13 Suze Kooij,14 Annemarie Slotboom,14 Marieke de Jong,15 Friedus van der Minne,15 Esther Santegoets.16
Study monitors
Leontien Heiligers1, Yvonne Martens,1 Naziha El Ghannouti.1
Affiliations
1Erasmus MC University Medical Center, Rotterdam, the Netherlands;
2Leiden University Medical Center, Leiden, the Netherlands;
3Amsterdam University Medical Centers, location AMC, Amsterdam, the Netherlands;
4Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre, Maastricht, The Netherlands;
5University Medical Center Utrecht, Brain Center Rudolf Magnus, Utrecht, the Netherlands;
6Catharina Hospital, Eindhoven, the Netherlands;
7Haaglanden Medical Centre, the Hague, the Netherlands;
8Rijnstate Hospital, Arnhem, the Netherlands;
9St. Antonius Hospital, Nieuwegein, the Netherlands;
10Radboud University Medical Center, Nijmegen, the Netherlands;
11HagaZiekenhuis, the Hague, the Netherlands;
12Isala, Zwolle, the Netherlands;
13Amphia Hospital, Breda, the Netherlands;
14Albert Schweitzer Hospital, Dordrecht, the Netherlands;
15University Medical Center Groningen, Groningen, the Netherlands;
16Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands;
17Lygature, Utrecht, the Netherlands;
18School for Mental Health and Sciences (Mhens), Maastricht University Medical Center, Maastricht, The Netherlands
19Texas Stroke Institute, Dallas-Fort Worth, Texas, United States of America.
20Medisch Spectrum Twente, Enschede, The Netherlands.
21TerGooi, Hilversum, The Netherlands.
For the MR CLEAN Registry:
Executive committee: Diederik W.J. Dippel1;Aad van der Lugt2;Charles B.L.M. Majoie3;Yvo B.W.E.M. Roos4;Robert J. van Oostenbrugge5;Wim H. van Zwam6;Jelis Boiten14;Jan Albert Vos.8
Study coordinators: Ivo G.H. Jansen3;Maxim J.H.L. Mulder1,2;Robert- Jan B. Goldhoorn5,6;Kars C.J. Compagne2;Manon Kappelhof3;Josje Brouwer4;Sanne J. den Hartog1,2,40;Wouter H. Hinsenveld 5,6;
Local principal investigators: Diederik W.J. Dippel1;Bob Roozenbeek1;Aad van der Lugt2;Adriaan C.G.M. van Es2;Charles B.L.M. Majoie3;Yvo B.W.E.M. Roos4;Bart J. Emmer3;Jonathan M. Coutinho4;Wouter J. Schonewille7;Jan Albert Vos8; Marieke J.H. Wermer9;Marianne A.A. van Walderveen10;Julie Staals5;Robert J. van Oostenbrugge5;Wim H. van Zwam6;Jeannette Hofmeijer11;Jasper M. Martens12;Geert J. Lycklama à Nijeholt13;Jelis Boiten14;Sebastiaan F. de Bruijn15;Lukas C. van Dijk16;H. Bart van der Worp17;Rob H. Lo18;Ewoud J. van Dijk19;Hieronymus D. Boogaarts20;J. de Vries22;Paul L.M. de Kort21; Julia van Tuijl21; Jo P. Peluso26;Puck Fransen22;Jan S.P. van den Berg22;Boudewijn A.A.M. van Hasselt23;Leo A.M. Aerden24;René J. Dallinga25;Maarten Uyttenboogaart28;Omid Eschgi29;Reinoud P.H. Bokkers29;Tobien H.C.M.L. Schreuder30;Roel J.J. Heijboer31;Koos Keizer32;Lonneke S.F. Yo33;Heleen M. den Hertog22;Emiel J.C. Sturm35; Paul J.A.M. Brouwers.34
Imaging assessment committee: Charles B.L.M. Majoie3(chair); Aad van der Lugt2(co-chair); Wim H. van Zwam6; Geert J. Lycklama à Nijeholt13;Marianne A.A. van Walderveen10;Marieke E.S. Sprengers3;Sjoerd F.M. Jenniskens27;René van den Berg3;Albert J. Yoo38;Ludo F.M. Beenen3;Alida A. Postma6;Stefan D. Roosendaal3;Bas F.W. van der Kallen13;Ido R. van den Wijngaard13;Adriaan C.G.M. van Es2;Bart J. Emmer,3;Jasper M. Martens12; Lonneke S.F. Yo33;Jan Albert Vos8; Joost Bot36, Pieter-Jan van Doormaal2; Anton Meijer27;Elyas Ghariq13; Reinoud P.H. Bokkers29;Marc P. van Proosdij37;G. Menno Krietemeijer33;Jo P. Peluso26;Hieronymus D. Boogaarts20;Rob Lo18;Dick Gerrits35;Wouter Dinkelaar2Auke P.A. Appelman29;Bas Hammer16;Sjoert Pegge27;Anouk van der Hoorn29;Saman Vinke20.
Writing committee: Diederik W.J. Dippel1(chair);Aad van der Lugt2;Charles B.L.M. Majoie3;Yvo B.W.E.M. Roos4;Robert J. van Oostenbrugge5;Wim H. van Zwam6;Geert J. Lycklama à Nijeholt13;Jelis Boiten14;Jan Albert Vos8;Wouter J. Schonewille7;Jeannette Hofmeijer11;Jasper M. Martens12;H. Bart van der Worp17;Rob H. Lo.18
Adverse event committee: Robert J. van Oostenbrugge5(chair); Jeannette Hofmeijer11; H. Zwenneke Flach.23
Trial methodologist: Hester F. Lingsma.40
Research nurses and local trial coordinators: Naziha el Ghannouti1;Martin Sterrenberg1;Wilma Pellikaan7;Rita Sprengers4;Marjan Elfrink11;Michelle Simons11;Marjolein Vossers12;Joke de Meris14;Tamara Vermeulen14;Annet Geerlings19;Gina van Vemde22;Tiny Simons30;Gert Messchendorp28;Nynke Nicolaij28;Hester Bongenaar32;Karin Bodde24;Sandra Kleijn34;Jasmijn Lodico34; Hanneke Droste34;Maureen Wollaert5;Sabrina Verheesen5;D. Jeurrissen5;Erna Bos9;Yvonne Drabbe15;Michelle Sandiman15;Nicoline Aaldering11;Berber Zweedijk17;Jocova Vervoort21;Eva Ponjee22;Sharon Romviel19;Karin Kanselaar19;Denn Barning10; Rita Sprengers3.
PhD and Medical students: Esmee Venema40; Vicky Chalos1,40; Ralph R. Geuskens3; Tim van Straaten19;Saliha Ergezen1; Roger R.M. Harmsma1; Daan Muijres1; Anouk de Jong1;Olvert A. Berkhemer1,3,6;Anna M.M. Boers3,39; J. Huguet3;P.F.C. Groot3;Marieke A. Mens3;Katinka R. van Kranendonk3;Kilian M. Treurniet3;Manon L. Tolhuisen3,39;Heitor Alves3;Annick J. Weterings3,Eleonora L.F. Kirkels3,Eva J.H.F. Voogd11;Lieve M. Schupp3;Sabine Collette28,29;Adrien E.D. Groot4;Natalie E. LeCouffe4;Praneeta R. Konduri39;Haryadi Prasetya39;Nerea Arrarte-Terreros39;Lucas A. Ramos39.
Department of Neurology1, Radiology2, Public Health40, Erasmus MC University Medical Center;
Department of Radiology and Nuclear Medicine3, Neurology4, Biomedical Engineering & Physics39, Amsterdam UMC, University of Amsterdam, Amsterdam;
Department of Neurology5, Radiology6, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM);
Department of Neurology7, Radiology8, Sint Antonius Hospital, Nieuwegein;
Department of Neurology9, Radiology10, Leiden University Medical Center;
Department of Neurology11, Radiology12, Rijnstate Hospital, Arnhem;
Department of Radiology13, Neurology14, Haaglanden MC, the Hague;
Department of Neurology15, Radiology16, HAGA Hospital, the Hague;
Department of Neurology17, Radiology18, University Medical Center Utrecht;
Department of Neurology19, Neurosurgery20, Radiology27, Radboud University Medical Center, Nijmegen;
Department of Neurology21, Radiology26, Elisabeth-TweeSteden ziekenhuis, Tilburg;
Department of Neurology22, Radiology23, Isala Klinieken, Zwolle;
Department of Neurology24, Radiology25, Reinier de Graaf Gasthuis, Delft;
Department of Neurology28, Radiology29, University Medical Center Groningen;
Department of Neurology30, Radiology31, Atrium Medical Center, Heerlen;
Department of Neurology32, Radiology33, Catharina Hospital, Eindhoven;
Department of Neurology34, Radiology35, Medical Spectrum Twente, Enschede;
Department of Radiology36, Amsterdam UMC, Vrije Universiteit van Amsterdam, Amsterdam;
Department of Radiology37, Noordwest Ziekenhuisgroep, Alkmaar;
Department of Radiology38, Texas Stroke Institute, Texas, United States of America.
CLEOPATRA Principal Investigators
Bart Emmer (MD, PhD)1, Charles Majoie (MD, PhD)1, Henk Marquering (PhD)1, Hugo de Jong (PhD)2, Hester Lingsma (PhD)3, Diederik Dippel (MD, PhD)3
Study coordinators
Arnolt-Jan Hoving (MD)1, Henk van Voorst (MD)1, Daan Peerlings (MSc)2, Jasper Daems (MD)3, Miou Koopman (MD)1
Local principal investigators (CONTRAST, MR CLEAN Registry, and CLEOPATRA)
Jonathan Coutinho (MD, PhD)1, Bart van der Worp (MD, PhD)2, Rob Lo (MD, PhD)2, Jan Willem Dankbaar (MD, PhD)2, Bob Roozenbeek (MD, PhD)3, Adriaan van Es (MD, PhD)3, Pieter-Jan van Doormaal (MD)3, Jasper Martens (MD)4, Jeanette Hofmeijer (MD, PhD)4, Maarten Uyttenboogaart (MD, PhD)5, Reinoud Bokkers (MD, PhD)5, Marianne van Walderveen (MD, PhD)6, Marieke Wermer (MD, PhD)6, Lonneke Yo (MD, PhD)7, Koos Keizer (MD, PhD)7, Rob Gons (MD)7, Aad van der Lugt (MD, PhD)3, Julie Staals (MD, PhD)8, Wim van Zwam (MD,PhD)8, Inger de Ridder (MD, PhD)8, Karlijn de Laat (MD, PhD)9, Lukas van Dijk (MD, PhD)9, Jelis Boiten (MD, PhD)10, Sebastiaan de Bruijn (MD, PhD)9, Geert Lycklama à Nijeholt (MD, PhD)10, Ido van den Wijngaard (MD, PhD)10, Paul de Kort (MD, PhD)11, Michel Remmers (MD)11, Anouk van Norden (MD)11, Issam Boukrab (MD)11, Julia van Tuijl (MD)11, Hans Kortman (MD)11, Anton Meijer (MD, PhD)12, Floris Schreuder (MD, PhD)12, Ewoud van Dijk (MD, PhD)12, Hieronymus Boogaarts (MD, PhD)12, Heleen den Hertog (MD, PhD)13, Zwenneke Flach (MD)13, Jan van den Berg (MD)13, Boudewijn van Hasselt (MD)13, Michel Remmers (MD)14, Anouk van Norden (MD)14, Farshad Imani (MD)14, Otto Elgersma (MD, PhD)15, Anouk Rozeman (MD)15, Paul Brouwers (MD, PhD)16, Dick Gerrits (MD)16, Thomas Bulut (MD)16, Paul Brouwers (MD)16, Jan Albert Vos (MD, PhD)17, Wouter Schonewille (MD, PhD)17
CLEOPATRA Advisory Board
Albert Yoo (MD, PhD)18, Wolfgang Kunz (MD, PhD)19, Bruce Campbell (MD, PhD)20
Trial Office Zorgevaluatie Nederland Nienke Pannekoek (PhD)21
Local trial collaborators:
Executive and writing committee
Bart Emmer (MD, PhD)1, Charles Majoie (MD, PhD)1, Erik Buskens (MD, PhD)5, Hester Lingsma (PhD)3, Henk Marquering (PhD)1, Hugo de Jong (PhD)2, Olvert Berkhemer (MD, PhD)1, Wim van Zwam (MD, PhD)8, Marianne van Walderveen (MD, PhD)6, Ido van den Wijngaard (MD, PhD)10, Aad van der Lugt (MD, PhD)3, Diederik Dippel (MD, PhD)3, Arnolt-Jan Hoving (MD)1, Henk van Voorst (MD)1, Daan Peerlings (MSc)2, Jasper Daems (MD)3
Trial statistician
Hester Lingsma (PhD)3
CONTRAST clinical trial collaborators:
CONTRAST research leaders
Diederik Dippel (MD, PhD)2, Aad van der Lugt (MD, PhD)2, Charles Majoie (MD, PhD)1, Yvo Roos (MD, PhD)1, Robert van Oostenbrugge (MD, PhD)8, Wim van Zwam (MD, PhD)8
CONTRAST Imaging assessment committee
Charles Majoie (MD, PhD)1 – Chair, Aad van der Lugt (MD, PhD)3 – Chair, Wim van Zwam (MD, PhD)8, Linda Jacobi (MD, PhD)8, René van den Berg, (MD, PhD)1, Ludo Beenen (MD, PhD)1, Bart Emmer (MD, PhD)1, Adriaan van Es, (MD, PhD)3, Pieter-Jan van Doormaal (MD)3, Geert Lycklama à Nijeholt (MD, PhD)10, Ido van den Wijngaard (MD, PhD)10, Albert Yoo (MD, PhD)18, Lonneke Yo (MD, PhD)7, Jasper Martens (MD, PhD)4, Bas Hammer (MD, PhD)9, Stefan Roosendaal (MD, PhD)1, Anton Meijer (MD, PhD)12, Menno Krietemeijer (MD)7, Reinoud Bokkers (MD, PhD)4, Anouk van der Hoorn (MD, PhD)4, Dick Gerrits (MD)16
MR CLEAN Registry Imaging assessment committee
Charles Majoie (MD, PhD)1 – Chair, Wim van Zwam8, Aad van der Lugt2; Geert Lycklama à Nijeholt (MD, PhD)10, Marianne van Walderveen (MD, PhD)6, Marieke Sprengers (MD, PhD)1; Sjoer Jenniskens (MD, PhD)12, René van den Berg (MD, PhD)1, Albert Yoo (MD, PhD)19 , Ludo Beenen (MD, PhD)1, Alida Postma (MD, PhD)8; Stefan Roosendaal (MD, PhD)1, Bas van der Kallen (MD, PhD)4, Ido van den Wijngaard (MD, PhD)10, Adriaan van Es (MD, PhD)3; Bart Emmer (MD, PhD)1, Jasper Martens (MD)4, Lonneke Yo (MD, PhD)7, Jan Albert Vos (MD, PhD)17, Joost Bot (MD, PhD)1; Pieter-Jan van Doormaal (MD, PhD)2, Anton Meijer (MD, PhD)12, Elyas Ghariq (MD)10, Reinoud Bokkers (MD, PhD)5, Menno Krietemeijer (MD)7, Hieronymus Boogaarts (MD, PhD)12, Rob Lo (MD, PhD)2, Wouter Dinkelaar (MD)15, Auke Appelman (MD, PhD)5; Bas Hammer (MD)9, Sjoert Pegge (MD)9, Anouk van der Hoorn (MD, PhD)4, Saman Vinke (MD, PhD)12; Sandra Cornelissen (MD)3, Christiaan van der Leij (MD)8, Rutger Brans (MD)8, Jeanette Bakker (MD, PhD)15, Maarten Uyttenboogaart (MD, PhD)5, Miou Koopman (MD, PhD)1, Lucas Smagge (MD, PhD)3, Olvert A. Berkhemer (MD, PhD)1, Jeroen Markenstein (MD, PhD)1; Eef Hendriks (MD, PhD)1, Patrick Brouwer (MD, PhD)6
Data management group
Bart Emmer (MD, PhD)1, Charles Majoie (MD, PhD)1, Henk van Voorst (MD)1, Arnolt-Jan Hoving (MD)1
CONTRAST imaging data management
Aad van der Lugt (MD, PhD)2, Charles Majoie (MD, PhD)1, Adriaan Versteeg2, Lennard Wolff (MD)2, Jiahang Su (MSc)2, Manon Tolhuisen (MSc)1, Henk van Voorst (MD)1
MR CLEAN Registry clinical/imaging data acquisition
Esmee Venema3, Vicky Chalos3, Ralph Geuskens1, Tim van Straaten12, Saliha Ergezen3, Roger Harmsma3, Daan Muijres3, Anouk de Jong3, Olvert Berkhemer1, Merel Boers1, J. Huguet1, Paul Groot1, Marieke Mens1, Katinka van Kranendonk1, Kilian Treurniet1, Manon Tolhuisen1, Heitor Alves1, Annick Weterings1, Eleonora Kirkels1, Eva Voogd4, Lieve Schupp1, Sabine L. Collette5, Adrien Groot1, Natalie LeCouffe1, Praneeta Konduri1, Haryadi Prasetya1, Nerea Arrarte Terreros1, Lucas Ramos1, Nikki Boodt3, Anne Pirson8, Agnetha Bruggeman1, Nadinda van der Ende3, Rabia Deniz1, Susan Olthuis8, Floor Pinckaers8
CLEOPATRA local collaborators
Arnolt-Jan Hoving (MD)1, Henk van Voorst (MD)1, Daan Peerlings (MSc)2, Jasper Daems (MD)3, Agnetha Bruggeman (MD)1, Miou Koopman (MD)1, Anke Wouters (MD, PhD)1, Floor Pinckaers (MD)8, Susan Olthuis (MD)8
CONTRAST local collaborators
Josje Brouwer (MD)1, Manon Kappelhof (MD, PhD)1, Vicky Chalos (MD)3, Kilian Treurniet (MD, PhD)1, Sophie van den Berg (MD)1, Natalie LeCouffe (MD)1, Rob van de Graaf (MD)2, Robert-Jan Goldhoorn (MD, PhD)4, Aladdin Taha (MD)3, Samantha Donse-Donkel (MD)3, Wouter Hinsenveld (MD)8, Anne Pirson (MD)8, Lotte Sondag (MD)10, Manon Kappelhof (MD, PhD)1, Rik Reinink (MD)5, Manon Tolhuisen (MD)1, Josje Brouwer (MD)1, Lennard Wolff (MD)3, Sabine Collette5, Wouter van der Steen (MD)3
Affiliations
1 Amsterdam University Medical Centers, location University of Amsterdam, Amsterdam the Netherlands; 2 University Medical Center Utrecht, Utrecht, the Netherlands; 3 Erasmus MC University Medical Center, Rotterdam, the Netherlands; 4 Rijnstate Hospital, Arnhem, the Netherlands; 5 University Medical Center Groningen, Groningen, the Netherlands; 6 Leiden University Medical Center, Leiden, the Netherlands; 7 Catharina Hospital, Eindhoven, the Netherlands; 8 Maastricht University Medical Center, Cardiovascular Research Institute Maastricht (CARIM), Maastricht, The Netherlands; 9 HagaZiekenhuis, the Hague, the Netherlands; 10 Haaglanden Medical Center, the Hague, the Netherlands; 11 Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; 12 Radboud University Medical Center, Nijmegen, the Netherlands; 13 Isala Klinieken, Zwolle, the Netherlands; 14 Amphia Hospital, Breda, the Netherlands; 15 Albert Schweitzer Hospital, Dordrecht, the Netherlands; 16 Medisch Spectrum Twente, Enschede, the Netherlands; 17 St. Antonius Hospital, Nieuwegein, the Netherlands; 18 Texas Stroke Institute, Plano, TX, USA; 19 University Hospital, LMU Munich, Munich, Germany; 20 Melbourne Brain Center, Royal Melbourne Hospital, University of Melbourne, Parkville, VIC, Australia; 21 Trialbureau Zorgevaluatie Nederland, Diemen, the Netherlands
Funding
This study was partly funded by the CONTRAST consortium and a grant from Leading the Change (ZonMW). The CONTRAST consortium is supported by Netherlands Cardiovascular Research Initiative (CVON), an initiative of the Dutch Heart Foundation, the Brain Foundation Netherlands, Medtronic and Cerenovus. The MR CLEAN Registry was partly funded by the TWIN Foundation and by Erasmus MC University Medical Center, Maastricht University Medical Center, and Amsterdam UMC.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor for this study is Charles BLM Majoie.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Codman, Stryker, AngioCare, Medtronic, Covidien, EV3, MEDAC, LAMePRO, Penumbra, Top Medical, Concentric, Nicolab, Medtronic, Cerenovus, Bracco Imaging, Servier, Genentech, Vesalio, Philips, Zoll Circulation, Insera Therapeutics.
WHvZ reports personal fees from Codman and from Stryker. DWJD report grants from the Dutch Heart Foundation, AngioCare, Medtronic/Covidien/EV3, MEDAC/LAMEPRO, Penumbra, Top Medical/Concentric, Stryker, and Cerenovus; consultation fees from Stryker, Bracco Imaging, and Servier, received by the Erasmus University Medical Centre outside this project. CBLMM reports grants from TWIN, during the conduct of the study and grants from CVON/Dutch Heart Foundation, European Commission, Dutch Health Evaluation Program, and from Stryker outside this project (paid to institution) and is shareholder of Nicolab. AJY reports Research grants from Medtronic, Cerenovus, Penumbra, Stryker, and Genentech. Consultant for Penumbra, Cerenovus, Nicolab, Philips, Vesalio, Zoll Circulation, and NIH/NINDS. YR is a shareholder of Nicolab. Equity interests in Insera Therapeutics and Nicolab. All other contributors report no conflicts of interest.
Statistics and biometry
One of the authors has significant statistical expertise: Erik Buskens, University Medical Center Groningen, Department of Epidemiology, coauthor of this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Patient inclusion was in adherence with the Declaration of Helsinki and was subject to an ethical board review. The CLEOPATRA study protocol has been reviewed by the Amsterdam UMC ethical review board and was waived for informed consent (internal reference: W19_281#19.334). Retrospective, large-scale, observational studies do not fall under the Medical Research Involving Human Subjects Act (WMO). For patients included in the MR CLEAN-NO IV (ISRCTN80619088, registered 31 October 2017), and MR CLEAN MED (ISRCTN76741621, registered 7 December 2017) trials, informed consent has been received previously. The ethical review board of the Erasmus MC has waived the requirement for informed consent for patients included in the MR CLEAN Registry (internal reference Erasmus MC: MEC-2014–235, 27 August 2014).
Study subjects or cohorts overlap
Of all 701 patients considered in this study, data from 622 have previously been reported. Patients were included in the MR CLEAN NO IV trial (n = 227), MR CLEAN MED trial (n = 117), and MR CLEAN Registry (n = 278). All included patients were selected as a subset of the overall MR CLEAN NO IV, MR CLEAN MED, and MR CLEAN Registry populations. We used patients included in these studies to extract population statistics (age, sex) and functional outcomes that, combined with literature, were used to simulate long-term functional outcome, costs, and QALYs. Based on simulations we describe the effect on costs and QALYs of various scenarios for screening patients with CTP. Current other studies did not have related research aims or hypotheses. We selected these patients based on the presence of CTP before EVT to describe a realistic cohort of patients that receive CTP. Patients included in the MR CLEAN NO IV (n = 227) and MR CLEAN MED (n = 117) have been studied in a randomized controlled trial setting for the effect of intravenous alteplase (0.9 mg/kg) before EVT and the effect of heparin and aspirin before endovascular treatment respectively. Functional outcome and population characteristics of the patients included MR CLEAN Registry (n = 278) have been reported before. A similar cost-effectiveness analysis for CTP based on the same population is under review. However, this other study considers CTP for EVT patient selection and not for EVT eligible occlusion detection.
There exists a pre-print, https://doi.org/10.1101/2023.03.16.23287253.
Methodology
-
Retrospective
-
Multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Voorst, H., Hoving, J.W., Koopman, M.S. et al. Cost-effectiveness of CT perfusion for the detection of large vessel occlusion acute ischemic stroke followed by endovascular treatment: a model-based health economic evaluation study. Eur Radiol 34, 2152–2167 (2024). https://doi.org/10.1007/s00330-023-10119-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10119-y