Abstract
Objectives
To evaluate the prognostic value of CT-based markers of sarcopenia and myosteatosis in comparison to the Eastern Cooperative Oncology Group (ECOG) score for survival of patients with advanced pancreatic cancer treated with high-intensity focused ultrasound (HIFU).
Materials and methods
For 142 retrospective patients, the skeletal muscle index (SMI), skeletal muscle radiodensity (SMRD), fatty muscle fraction (FMF), and intermuscular fat fraction (IMFF) were determined on superior mesenteric artery level in pre-interventional CT. Each marker was tested for associations with sex, age, body mass index (BMI), and ECOG. The prognostic value of the markers was examined in Kaplan-Meier analyses with the log-rank test and in uni- and multivariable Cox proportional hazards (CPH) models.
Results
The following significant associations were observed: Male patients had higher BMI and SMI. Patients with lower ECOG had lower BMI and SMI. Patients with BMI lower than 21.8 kg/m2 (median) also showed lower SMI and IMFF. Patients younger than 63.3 years (median) were found to have higher SMRD, lower FMF, and lower IMFF. In the Kaplan-Meier analysis, significantly lower survival times were observed in patients with higher ECOG or lower SMI. Increased patient risk was observed for higher ECOG, lower BMI, and lower SMI in univariable CPH analyses for 1-, 2-, and 3-year survival. Multivariable CPH analysis for 1-year survival revealed increased patient risk for higher ECOG, lower SMI, lower IMFF, and higher FMF. In multivariable analysis for 2- and 3-year survival, only ECOG and FMF remained significant.
Conclusion
CT-based markers of sarcopenia and myosteatosis show a prognostic value for assessment of survival in advanced pancreatic cancer patients undergoing HIFU therapy.
Clinical relevance statement
The results indicate a greater role of myosteatosis for additional risk assessment beyond clinical scores, as only FMF was associated with long-term survival in multivariable CPH analyses along ECOG and also showed independence to ECOG in group analysis.
Key Points
• This study investigates the prognostic value of CT-based markers of sarcopenia and myosteatosis for patients with pancreatic cancer treated with high-intensity focused ultrasound.
• Markers for sarcopenia and myosteatosis showed a prognostic value besides clinical assessment of the physical status by the Eastern Cooperative Oncology Group score. In contrast to muscle size measurements, the myosteatosis marker fatty muscle fraction demonstrated independence to the clinical score.
• The results indicate that myosteatosis might play a greater role for additional patient risk assessments beyond clinical assessments of physical status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is an oncologic disease with a very poor prognosis and an estimated 5-year survival rate of below 10%. Surgical resection can cure pancreatic cancer in early stage; however, the majority of patients are already unresectable at initial diagnosis [1, 2]. Advanced pancreatic cancer is often associated with a very poor quality of life due to cancer pain and a very short life expectancy despite current oncological treatment with chemotherapy or chemoradiotherapy [1]. Local ablation with minimal invasive high-intensity focused ultrasound (HIFU) is an additional treatment option that is often combined with palliative standard treatment, e.g., systemic chemotherapy. With this technique, therapeutic ultrasound (US) waves are focused on the pancreatic lesion to induce coagulative necrosis, leaving healthy tissue outside the focus unharmed. HIFU treatment has been shown to reduce disease-associated symptoms, e.g., cancer pain or tumor mass, and to prolong the survival of patients compared to patients undergoing chemotherapy alone [1, 3, 4].
In patients with pancreatic cancer, a multifactorial syndrome, termed cancer cachexia, is particularly common [5]. This syndrome is induced by reduction of nutritional intake (e.g., due to cancer pain, fatigue, depression, insufficiency of pancreatic enzymes, or side effects of chemotherapy, e.g., nausea and vomiting) and an elevated energy metabolism (e.g., due to increased glucose and protein turnover because of advanced cancer) [2, 6]. Cancer cachexia is associated with ongoing loss of weight, skeletal muscle mass (termed sarcopenia), and physical performance leading to reduced quality of life and life expectancy [2, 5]. An established clinical score for evaluating the physical performance and general physical condition of patients is the Eastern Cooperative Oncology Group (ECOG) performance status that describes how limited the patient is in work activity, self-care, and his walking ability [7]. Evaluating the general physical condition by ECOG showed a strong prognostic value for outcome assessments in pancreatic and other cancer patients [8, 9].
Patients with advanced pancreatic cancer usually receive CT examinations for staging purposes prior to initiation of treatment and during therapy. Besides the diagnostic intention, these CT scans can also be utilized opportunistically to assess the patient’s constitution. Here, muscle size measurements are typically used as a surrogate marker for assessment of muscle wasting in sarcopenia. Additionally, muscle radiodensity evaluations are used to measure infiltration of lipids into the intra- and intermyocellular compartments, termed myosteatosis [10,11,12,13]. This opportunistic approach is also driven by recent successes of deep learning in medical imaging, which increases the clinical applicability of image-based analysis by automating otherwise time-consuming tissue segmentations [14,15,16]. To date, the prognostic value of imaging-based assessment of sarcopenia and myosteatosis has been demonstrated for patients with pancreatic cancer receiving chemotherapy or surgical resection, but not for patients undergoing HIFU therapy [2, 10, 17,18,19]. For instance, a meta-analysis of multiple studies on body composition and sarcopenia observed significant overall effects for sarcopenia evaluations based on muscle size measurements in CT imaging of patients with resectable and unresectable pancreatic cancer [17]. Another study additionally observed a prognostic value of radiodensity measurements of the muscles in CT for the evaluation of myosteatosis in patients with pancreatic cancer treated with palliative chemotherapy [10].
Therefore, the aim of this study was to evaluate the prognostic value of CT-based assessment of sarcopenia and myosteatosis in comparison to clinical assessment of physical status by ECOG for survival prediction in patients with advanced pancreatic cancer undergoing local US-guided HIFU ablation. Additionally, this study aims to investigate associations between the image-based markers, ECOG, and basic clinical parameters.
Material and methods
With the approval of institutional review board of the Medical Faculty of the Rheinische Friedrich-Wilhelms-Universitat Bonn, written informed consent was waived due to the retrospective, single-center nature of the study. The study was carried out in compliance with the ethical standards set in the 1964 Declaration of Helsinki as well as its later amendments. Consecutive patients with advanced pancreatic adenocarcinoma undergoing local US-guided HIFU treatment at our center between May 2014 and April 2020 and available CT within 14 days prior to intervention were included. Sex, age, body mass index (BMI), and ECOG were assessed from the clinical data system. The musculus erector spinae was segmented on an axial CT slice at the level of the superior mesenteric artery by a deep learning method [15]. The automatic segmentation was then manually optimized by a medical resident and finally approved by a board-certified radiologist.
CT-based markers of sarcopenia and myosteatosis
Figure 1 illustrates the computation of image-based markers in detail. To assess muscle size, the established “skeletal muscle index” (SMI) was calculated from the total area of muscle compartment and body height, as frequently applied in muscle-based body composition analysis [2, 10, 12, 18, 20]. For myosteatosis assessment, the two previously proposed markers “skeletal muscle radiodensity” (SMRD) and “fatty muscle fraction” (FMF) were determined [10, 13]. FMF aims to quantify the extent of intramuscular fat infiltration by relating the area of fatty degenerated muscle to the combined area of lean muscle and fatty degenerated muscle. To additionally assess intermuscular fat not captured by SMRD and FMF, the percentage of pure intermuscular fat tissue was extracted as “intermuscular fat fraction” (IMFF).
Overview of image-based markers. On the left, a transversal CT scan slice at the level of superior mesenteric artery is shown. The musculus erector spinae was segmented applying a deep learning model. If necessary, automatic segmentations were manually optimized. The segmented area was subdivided into different tissue classes using different ranges of Hounsfield units (HU). The skeletal muscle index (SMI), skeletal muscle radiodensity (SMRD), fatty muscle fraction (FMF), and intermuscular fat fraction (IMFF) were calculated according to the definitions shown on the right
Statistical analysis
First, each CT marker was tested for associations with clinical attributes. Therefore, the patients were divided into subgroups for sex, age and BMI (split by the respective median value), ECOG (score 0, 1, and ≥ 2), and the survival status after 1 and 2 years. For each subgroup, median and 25th and 75th interquartile ranges (IQR) are provided. Differences between the subgroups were assessed by the Mann-Whitney U test for sex, age, BMI, and survival status, and the Kruskal-Wallis H test for ECOG (SciPy 1.8.0) [21]. Differences with p value < 0.05 were considered significant.
Then, differences in survival time between subgroups split by clinical and image-based markers were examined in the Kaplan-Meier analysis with the log-rank test. For this, all continuous parameters, i.e., all parameters except sex and ECOG, were divided into subgroups according to sex-specific median values.
Finally, all clinical attributes and imaging markers were examined in univariable CPH models as well as in multivariable CPH models including all parameters. Kaplan-Meier and CPH analyses were conducted with SPSS (27.0.0, IBM).
Results
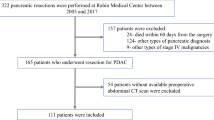
Prior to analysis, 153 eligible patients treated with HIFU were identified. Eleven patients were excluded because no CT scan was acquired within 14 days prior to intervention, or due to missing body height or weight records at time of CT imaging. Therefore, a total of 142 patients (73 females, mean age 64.1 ± 10.5 years, range 38–87.5) were included for analysis. Table 1 shows detailed clinical characteristics of the patients included.
Median values and interquartile ranges for age, BMI, SMI, SMRD, FMF, and IMFF split into subgroups by sex, median age, median BMI, survival status after 1 and 2 years, and ECOG, are presented in Table 2. The following significant associations were observed: Male patients showed higher BMI and SMI. Patients with lower ECOG score had higher BMI and higher SMI. Patients with BMI higher than 21.8 kg/m2 (median) were observed to have higher SMI and higher IMFF. Patients older than 63.3 years (median) showed lower SMRD, higher FMF, and higher IMFF. Patients who survived 1 year had higher BMI and SMI, and patients who survived 2 years had higher BMI compared to patients who died earlier. Figure 2 illustrates violin and boxplots for SMI, BMI, and IMFF split by sex, ECOG score, median age, and median BMI.
Violin and boxplots for the muscle size assessing the skeletal muscle index (SMI), as well as for myosteatosis assessing markers skeletal muscle radio density (SMRD), fatty muscle fraction (FMF), and intermuscular fat fraction (IMFF) separated for females and males, separated at median age, separated at median body mass index (BMI), and separated for Eastern Cooperative Oncology Group performance status (ECOG). Differences between sexes, age, and BMI were tested using a two-tailed t test and between ECOG with Kruskal-Wallis H test, with significance indicated by an asterisk (***p values ≤ 0.001, **p values ≤ 0.01, *p values ≤ 0.05)
Table 3 shows 1-, 2-, and 3-year survival and the results of the Kaplan-Meier analysis with log-rank test for patients’ subgroups split by clinical and imaging-based parameters. Overall, the median survival time of all patients was 185 (IQR: 99–404) days. Only 10 patients survived longer than 2 years. Patients with low SMI and patients with higher ECOG score had lower survival times. Figure 3 shows the corresponding 3-year Kaplan-Meier survival curves. Hazard ratios and p values of the univariable and multivariable CPH models are shown in Table 4. Univariable CPH analyses for 1-, 2-, and 3-year survival showed that higher ECOG score, lower BMI, and lower SMI were associated with increased patient risk. Combining all parameters in multivariable CPH analyses for 1-year survival revealed that higher ECOG score, lower SMI, lower IMFF, and higher FMF were associated with increased patient risk. When parameters are examined in multivariable CPH models for 2- and 3-year survival, only ECOG and FMF remained significant.
The Kaplan-Meier curves for the 3-year survival of patients separated by the Eastern Cooperative Oncology Group performance status (ECOG) and by sex-specific median of the skeletal muscle index (SMI). Differences between groups were tested by the log-rank test and significance is indicated by an asterisk (***p values ≤ 0.001, **p values ≤ 0.01)
Discussion
This study investigates the prognostic value of CT imaging markers for sarcopenia and myosteatosis in comparison to clinical assessment of physical status by the ECOG score in patients with advanced pancreatic cancer treated with local US-guided HIFU ablation in addition to other palliative oncological treatments.
The results demonstrate that the ECOG performance status is a strong predictor of patient survival following HIFU therapy in both, Kaplan-Meier and CPH analyses. Previous studies have already demonstrated that the assessment of the patient’s general physical condition by the ECOG score has a strong prognostic value for pancreatic cancer patients treated with chemo- or chemoradiotherapy and for other cancer patients [8, 9]. Interestingly, other previous studies that evaluated ECOG for pancreatic cancer patients treated with chemotherapy and/or surgical resection did not observe such a strong prognostic value [18, 19]. However, only two ECOG groups were considered in these studies (ECOG = 0 and ≥ 1). This indicates that particularly a score of ECOG ≥ 2 may be associated with increased mortality.
We investigated SMI, as this marker already demonstrated a prognostic value for patients with pancreatic cancer treated by surgical resection and for various other oncological diseases in previous studies [17, 19, 20, 22, 23]. In the subgroup analysis, SMI showed a significant correlation to BMI. The significant hazard ratios of BMI and SMI in the univariable CPH analysis, their lower prognostic value in the multivariable CPH analyses, and their observed associations with ECOG suggest that part of the information of BMI and SMI is already well reflected by ECOG. However, the significant differences in SMI between patients who survived and patients who died within 1 year, along with the prognostic value of SMI in Kaplan-Meier and in the multivariable CPH analysis for 1-year survival, indicate that image-based assessment of muscle size on CT imaging prior to HIFU treatment provides additional information particularly for short-term survival.
In addition to quantification of muscle size by SMI, which is typically used for image-based assessment of sarcopenia, we also investigated markers assessing myosteatosis. Of note, in contrast to SMI, all myosteatosis markers were associated with age. The increase of myosteatosis with age was also described in previous studies [11]. The markers for myosteatosis showed no associations with the ECOG performance score, sex nor BMI, while SMI and BMI were associated with sex and ECOG.
Mean radiodensity of the musculature is a myosteatosis marker that showed prognostic relevance within univariable CPH analyses in a previous study of patients with unresectable pancreatic cancer treated with palliative chemotherapy [10]. However, for the patient cohort of our study, no prognostic value of SMRD was observed in Kaplan-Meier nor CPH analyses.
Besides SMI and SMRD, we also investigated the two markers FMF and IMFF explicitly aimed at assessing inter- and intramuscular fat infiltration in myosteatosis. These two markers did not show any prognostic value when considered in Kaplan-Meier or univariable CPH analysis alone. However, both markers were significant predictors along with SMI when combined with clinical parameters in multivariable CPH analysis for 1-year survival. Furthermore, FMF was the only image-based marker that retained predictive value along with ECOG in the multivariable Cox models for 2- and 3-year survival.
Interestingly, IMFF was observed as a protective predictor with hazard ratios below one, in contrast to FMF, for which patient risk increases with higher values. Due to the observed association of the protective predictor IMFF with BMI, it may be assumed that larger intermuscular fat depots represented a better nutritional status that prolongs short-term survival in the current cohort.
As described in other studies, the results of our study also underscore that sarcopenia and myosteatosis are not synonymous and that assessment of myosteatosis has the potential to provide important additional information [11].
Conclusion
In conclusion, this study demonstrates that image-based markers of sarcopenia and myosteatosis derived from pre-therapeutic CT scans have a prognostic value for patients with advanced pancreatic cancer after palliative HIFU therapy. Image-based assessment of myosteatosis might play a greater role in the evaluation of a patient’s physical status along with the established ECOG score than simple muscle size measurements.
Abbreviations
- BMI:
-
Body mass index
- CPH:
-
Cox proportional hazards
- ECOG:
-
Eastern Cooperative Oncology Group
- FMF:
-
Fatty muscle fraction
- HIFU:
-
High-intensity focused ultrasound
- IMFF:
-
Intermuscular fat fraction
- IQR:
-
Interquartile range
- SMI:
-
Skeletal muscle index
- SMRD:
-
Skeletal muscle radiodensity
- US:
-
Ultrasound
References
Sofuni A, Asai Y, Mukai S, Yamamoto K, Itoi T (2022) High-intensity focused ultrasound therapy for pancreatic cancer. J Med Ultrason. https://doi.org/10.1007/s10396-022-01208-4
Naumann P, Eberlein J, Farnia B et al (2019) Cachectic body composition and inflammatory markers portend a poor prognosis in patients with locally advanced pancreatic cancer treated with chemoradiation. Cancers 11(11):1655
Marinova M, Huxold HC, Henseler J et al (2019) Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic cancer. Ultraschall Med 40(05):625–637
Marinova M, Feradova H, Gonzalez-Carmona MA et al (2021) Improving quality of life in pancreatic cancer patients following high-intensity focused ultrasound (HIFU) in two European centers. Eur Radiol 31(8):5818–5829
Dhanapal R, Saraswathi TR, Govind RN (2011) Cancer cachexia. J Oral Maxillofac Pathol 15(3):257–260
Fearon KC, Moses AG (2002) Cancer cachexia. Int J Cardiol 85(1):73–81
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–656
Kalser MH, Barkin J, Macintyre JM (1985) Pancreatic cancer. Assessment of prognosis by clinical presentation. Cancer 56(2):397–402
Demirelli B, Babacan NA, Ercelep Ö et al (2021) Modified Glasgow prognostic score, prognostic nutritional index and ECOG performance score predicts survival better than sarcopenia, cachexia and some inflammatory indices in metastatic gastric cancer. Nutr Cancer 73(2):230–238
Rollins KE, Tewari N, Ackner A et al (2016) The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr 35(5):1103–1109
Correa-de-Araujo R, Addison O, Miljkovic I et al (2020) Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front Physiol 11:963
Murray TE, Williams D, Lee MJ (2017) Osteoporosis, obesity, and sarcopenia on abdominal CT: a review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist. Abdom Radiol (NY) 42(9):2376–2238
Luetkens JA, Faron A, Geissler HL et al (2020) Opportunistic computed tomography imaging for the assessment of fatty muscle fraction predicts outcome in patients undergoing transcatheter aortic valve replacement. Circulation 141(3):234–236
Magudia K, Bridge CP, Bay CP et al (2021) Population-scale CT-based body composition analysis of a large outpatient population using deep learning to derive age-, sex-, and race-specific reference curves. Radiology 298(2):319–329
Nowak S, Faron A, Luetkens JA et al (2020) Fully automated segmentation of connective tissue compartments for CT-based body composition analysis: a deep learning approach. Invest Radiol 55(6):357–366
Nowak S, Theis M, Wichtmann BD et al (2022) End-to-end automated body composition analyses with integrated quality control for opportunistic assessment of sarcopenia in CT. Eur Radiol 32(5):3142–3151
Bundred J, Kamarajah SK, Roberts KJ (2019) Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford) 21(12):1603–1612
Basile D, Parnofiello A, Vitale MG et al (2019) The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. J Cachexia Sarcopenia Muscle 10(2):368–377
Sugimoto M, Farnell MB, Nagorney DM et al (2018) Decreased skeletal muscle volume is a predictive factor for poorer survival in patients undergoing surgical resection for pancreatic ductal adenocarcinoma. J Gastrointest Surg 22(5):831–839
Faron A, Opheys NS, Nowak S et al (2021) Deep learning-based body composition analysis predicts outcome in melanoma patients treated with immune checkpoint inhibitors. Diagnostics 11(12):2314
Virtanen P, Gommers R, Oliphant TE et al (2020) SciPy 10: fundamental algorithms for scientific computing in Python. Nat Methods 17(3):261–272
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9(7):629–635
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57:58–67
Funding
Open Access funding enabled and organized by Projekt DEAL. S.N. was funded over a part of the study duration by RACOON (NUM), which is supported by the Federal Ministry of Education and Research of Germany under BMBF grant number 01KX2021. The funders had no influence on the conceptualization and design of the study, data analysis and data collection, preparation of the manuscript, and the decision to publish.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is PD Dr. med. Julian Luetkens.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board (University of Bonn).
Ethical approval
Due to the single-center and retrospective nature of the study, it was approved by the institutional review board of the Medical Faculty of the Rheinische Friedrich-Wilhelms-Universitat Bonn with waiver of written informed consent.
Study subjects or cohorts overlap
Some study subjects were included in a previous study. The aim of this previous study was to evaluate if a HIFU-induced early sterile inflammatory reaction is initiated after ablation of uterine fibroids and pancreatic carcinoma. The research question of the current submitted study has no overlap to this previous investigation (DOI: 10.1080/02656736.2021.1900926).
Methodology
-
retrospective
-
prognostic study
-
performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nowak, S., Kloth, C., Theis, M. et al. Deep learning–based assessment of CT markers of sarcopenia and myosteatosis for outcome assessment in patients with advanced pancreatic cancer after high-intensity focused ultrasound treatment. Eur Radiol 34, 279–286 (2024). https://doi.org/10.1007/s00330-023-09974-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09974-6