Abstract
Objectives
To define requirements that condition trust in artificial intelligence (AI) as clinical decision support in radiology from the perspective of various stakeholders and to explore ways to fulfil these requirements.
Methods
Semi-structured interviews were conducted with twenty-five respondents—nineteen directly involved in the development, implementation, or use of AI applications in radiology and six working with AI in other areas of healthcare. We designed the questions to explore three themes: development and use of AI, professional decision-making, and management and organizational procedures connected to AI. The transcribed interviews were analysed in an iterative coding process from open coding to theoretically informed thematic coding.
Results
We identified four aspects of trust that relate to reliability, transparency, quality verification, and inter-organizational compatibility. These aspects fall under the categories of substantial and procedural requirements.
Conclusions
Development of appropriate levels of trust in AI in healthcare is complex and encompasses multiple dimensions of requirements. Various stakeholders will have to be involved in developing AI solutions for healthcare and radiology to fulfil these requirements.
Clinical relevance statement
For AI to achieve advances in radiology, it must be given the opportunity to support, rather than replace, human expertise. Support requires trust. Identification of aspects and conditions for trust allows developing AI implementation strategies that facilitate advancing the field.
Key Points
• Dimensions of procedural and substantial demands that need to be fulfilled to foster appropriate levels of trust in AI in healthcare are conditioned on aspects related to reliability, transparency, quality verification, and inter-organizational compatibility.
•Creating the conditions for trust to emerge requires the involvement of various stakeholders, who will have to compensate the problem’s inherent complexity by finding and promoting well-defined solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
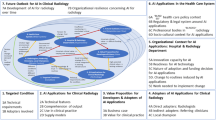
Using computer-based decision support systems in healthcare raises several issues, many of which fit the category of trust and trustworthiness. Specifically, it is imperative for professional stakeholders to develop trust in the efficacy of a system for its implementation to succeed [1]. Classical AI models pose a particular challenge in this regard. Their non-deterministic and correlational—rather than causal—nature results in the “black box” problem: the user has no means of scrutinizing the system’s decision process [2, 3]. A scoping review on the future of AI in radiology concluded that a majority of stakeholders disagree with the technocratic prospect of AI replacing human radiologists, and it identified trust as one of the seven determiners of success of AI in radiology [4]. Despite trust being a core requirement for AI in healthcare, little scientific work addresses trust. Gille et al [5] found no consensus on what trust is and how to achieve it in healthcare.
Few studies address the broad issue of trust in AI for medical imaging [6,7,8,9]. They focus mainly on the explainability and interpretability of algorithms as a requirement of trustworthy AI [6, 8, 9]. The common demand for AI models is to be explainable and interpretable so that human experts can understand the reasons for the model output [10]. The previous studies, while providing technical grounds for improving the trustworthiness of an algorithm, do not encompass medical reasoning in the explanations [11].
A broader look at trust and trustworthiness in relation to AI in medical image analysis support could provide grounds for healthcare professionals and other stakeholders to develop appropriate levels of trust towards AI. Hasani et al [7] proposed comprehensive requirements for developing trustworthy AI systems, including stakeholder engagement. They did not, however, cite empirical work in support of this requirement.
Trust depends on the interaction between the involved parties and should be understood as an ongoing process of establishing faith to reduce complexity [12, 13]. The social context is important for the interplay between a trustor and a trustee and consists of activities and strategies that will increase confidence between the involved parties [14]. Human actors come to trust each other or an AI system because of the role a trustee plays in the larger system, such as the organization [15]. Examples of interactive activities to establish propensity to trust [16] are (1) signalling of ability, (2) the demonstration of benevolence, (3) the demarcation of integrity, and (4) the establishment of an emotional connection [17]. Even though emotional connections are important for trust on an interpersonal level, AI in itself should not need to be trustworthy on an emotional level but reflect reliability on the same level as other technologies supporting medical decisions [18].
Taking a starting point in the scarceness of implementations of AI solutions for automatic segmentation of brain lesions on magnetic resonance images in clinical routine [19,20,21,22,23], the purpose of our study was to explore the knowledge gaps surrounding the broad themes of trust in AI, the perspective of stakeholders in AI, and how we can achieve trust in AI in healthcare. We designed an interview study covering a broad variety of stakeholders at one of Europe’s largest university hospitals and collaborating entities. We further aimed to define prerequisites for trust and to identify potential obstacles to achieving trust in healthcare when focusing on AI.
Materials and methods
Since AI in radiology can be considered a non-typical case [24], a purposive sampling strategy was used. We chose an explorative approach with a focus on how the ongoing development of AI opens for future changes [25]. We aimed to include a variety of respondents with a view to clinical and academic background (medical, technical, and administrative), workplace size, and geographical location. A chart representing demographic information on the 25 individuals participating in the interviews of this study and those invited but not participating (n = 13) is given in Fig. 1. The 25 participants held diverse roles in the healthcare system and most medical professionals also held (or had previously held) management or leadership functions and had academic backgrounds. Nineteen of the respondents were directly involved in the development, implementation, or use of AI applications as part of their professional practice related to radiology. Out of 38 invited respondents, 13 did not participate; two radiologists declined the invitation due to lack of time and one radiologist due to leave of absence. In addition, two radiologists, three neurosurgeons, two oncologists, one manager, and one MR-nurse did not respond to the invitation. One manager had resigned from work.
Data collection
The semi-structured interviews followed an interview guide (Supplement 1) with predefined questions allowing for the possibility to explore unanticipated issues that arose in connection with the data collection [26]. The interviews focused mainly on three themes—development and use of AI, professional decision-making, and management and organization—and included probing questions based on the participant’s responses [27].
The first interview topic consisted of questions on how different types of AI are used or are expected to be used by radiologists. We guided the respondents to stay close to their personal experiences and activities while mapping their use or expectations of AI and challenges related to the specific use of this technology.
The second topic addressed decision-making concerning responsibilities that condition the professional role. The questions pertained to AI and automation of state-of-the-art knowledge, standards, and skills central to the ability to address demands for accuracy, e.g., expert judgment on clinical matters and normative content. The interviews also addressed healthcare professionals’ responsibilities to comply with ethics, standards, and codes regulating their practice as recognized experts [28]. This part of the interview included questions about ambiguities related to accountability and public expectations on clinical reasoning, diagnostic work, and prioritization aligned with broader societal values or perceived common goods.
The third topic of the guide included questions about management and organizational procedures that condition the introduction of automated decision-making (ADM) into professional practice. We focused on the organizational goals and evaluations of administrative efficiency, fairness, quality, and safety issues linked with ADM. These questions were of interest in relation to many previous studies showing how managerial issues lead to the marginalization of professionals’ ability to make informed judgments [29]. By asking managers how they frame ADM, we intended to identify how organizational conditions shaped the ability to translate knowledge, codes, and standards to the needs and features of the case at hand [28]. We were thus able to identify further ambiguities conditioning professional discretionary capabilities.
The interviews were performed by two social scientists—not earlier working with specific neuroradiology-related questions to decrease interpretation bias (M.B. and B.R.)—recorded, and transcribed by an external transcriptionist. A logbook was kept in connection with each interview to record the investigators’ initial impression of the data.
Data analysis
We used the ATLAS.ti Web, Scientific Software Development GmbH (https://atlasti.com/) (AI add-ons recently available for the software were not used in this study) to identify, retrieve, and reflect on statements in the transcripts, applying and clustering codes in an iterative three-phase coding procedure (Fig. 2). In the first coding round, we kept close to the interviewees’ actual statements using concrete empirical and in vivo codes. In the second round of coding, we aggregated existing codes to identify how the range of activities involving AI was linked with broader clusters of meaning related to professional and organizational norms, values, rules, and policies. During this round of analysis, we identified themes linked with substantial dimensions of clinical work and procedural challenges. The third round of coding involved a re-reading of codes and themes based on theoretical reflection.
Coding procedure leading to the identification of conditions for trust in AI. In the first round, open coding was performed by tagging of words/text fragments in the input transcript data with concrete empirical and in vivo codes. In the second round of analysis (thematic coding), we aggregated existing codes to identify how the range of activities involving AI was linked with broader clusters of meaning related to professional and organizational norms, values, rules, and policies. During this round of analysis, we identified themes linked with substantial dimensions of clinical work and procedural challenges. In the third round (theoretically informed thematic coding), we iteratively re-read codes, text fragments identified in the previous rounds, and the transcripts. Thereby, we established theoretically informed themes leading to identification of conditions for trust in AI. The examples of raw data quotes, and more information on the identified theoretically informed themes and related conditions for trust in AI are given in Tables 1, 2, 3, and 4
Results
Of 912 coded text segments, 265 were directly related to aspects of trust. The iterative three-phase coding process is illustrated in Fig. 2. During open coding, concrete empirical and in vivo codes were defined e.g., visualizing, screening, segmentation, detecting, teleworking, free text, data sharing, managing data, training AI, mapping patterns, and decision support. The second coding round—thematic coding—resulted in identified themes linked with substantial dimensions of clinical work, e.g., judgment, ethics, demands for precision, exploration, skills, and accountability, and themes linked with procedural challenges, e.g., importance of standardisation, rationalisation, governance, and efficiency. In the second thematic coding round of the analysis, we identified trust as a recurring theme that emerged both on a local level in the radiologists’ practice and on a central organizational level connected to managerial and organizational demands.
The analysis of the interviews resulted in four theoretically informed themes of trust: trust in relation to reliability (64 codes); trust in relation to transparency (61 codes); trust in relation to quality verification (59 codes); trust in relation to inter-organizational compatibility (81 codes). The themes fit in two dimensions of trust: i.e., trust in substantial requirements and trust in procedural requirements. Substantial trust relates to trust in data, methods, infrastructure, and the like. Procedural trust relates to requirements that raise technical, organizational, and administrative challenges.
In Tables 1, 2, 3, and 4, we present and define the conditions under which the constituent aspects of four themes of trust generate trust in practice for our interviewees. The four themes are trust in relation to reliability, trust in relation to transparency, trust in relation to quality verification, and trust in relation to inter-organizational compatibility. Each aspect is supported by a quote from the interviews as an example of our definition of trust in practice.
Discussion
We identified four themes related to trust that are classified as substantial or procedural requirements. Developing solutions to the requirements demands participation from all stakeholders, in particular professionals using the technology. We further need to foster an organizational awareness of the importance of trust and collaboration of developers, users, regulators, and managers [5, 12]. As clinical implementation of AI in radiology is in its infancy, we must address concerns about developing appropriate levels of trust in AI to allow well-balanced clinical decisions based on automatically generated information [30, 31]. Developing such trust forces radiologists and other healthcare specialists to reflect on the consequences of including AI in professional judgment and decision-making in clinical practice, for instance, when AI solutions use combinations of retrospective and real-time health data to support evidence-based decision-making, individualized care, and precision medicine [32,33,34].
The reliability of AI is crucial to trust. We identified three aspects of reliability: volume, granularity, and bias. When examining large volumes of data, AI is expected to provide a dependable basis for diagnostics [35]. Access to increasing amounts of image data can create better diagnoses, but there is also a risk of information overflow. Reliability is generated when AI systematically returns predictable output in a large dataset. Granularity refers to how increased depth of information could result in higher precision in detecting findings given available resources to process and analyse the data. For example, the technological advances in imaging modalities lead to increased resolution or new types of available diagnostic images [36]. Those technological improvements can benefit patients only if the detailed information can be processed and analysed promptly. AI’s ability to accurately extract clinically relevant information from highly detailed information increases its reliability. The third identified reliability aspect is bias, i.e., the risk of being misled by preconceptions. AI’s ability to compare the current case with all existing reference cases increased the radiologist’s awareness of possible cognitive bias in decision-making [37]. By providing a second opinion, AI made the radiologist aware of potential bias. An example given by a radiologist in the interviews was that the more recent cases tended to influence them the most, whereas the AI considered all cases it had been trained on and thus provided them with a more extensive frame of reference [38].
Trust based on transparency draws on the radiologists’ understanding of the AI’s “inner workings” when handling individual cases [38]. We identified three themes crucial to transparency: standards, traceability, and explainability. Standards refer to how the AI can connect different cases to enhance the radiologists’ understanding of how data is managed so that the output becomes transferable to new cases. Standards make it possible to transfer insights from one case to another by providing evidence-based support that minimizes bias due to differences in competence and degree of experience. When AI becomes a trusted standard, we expect that the quality of diagnostics will improve in general. Traceability was an inherent aspect of standards as an interviewed radiologist argued that to be able to trust how the algorithm is processing data, the basis for making a decision must be traceable by domain professionals [39]. The requirements for standards and traceability lead to the third identified theme related to transparency: explainability. Explainability, defined as the ability of an AI system to provide a clear and understandable explanation of how it reached a particular decision or conclusion [40], enhances the increased diagnostic ability of radiologists as an informed interaction of humans and medical AI [41].
Various AI applications may require various degrees of trust towards the tool. Both traceability and explainability may be particularly important in scenarios, where the prediction of AI cannot be easily verified. For example, when AI is used for segmentation, physicians likely do not need the same degree of trust towards the tool since the outcome can be visually assessed. However, if for example dataset or distribution shift is present, it may not be feasible or even possible for the individual physician to verify the accuracy of the outcome to the same extent. Instead, physicians must develop appropriate levels of trust towards the support system. Therefore, other validation strategies based on traceability and explainability of the system are necessary to develop appropriate levels of trust towards AI.
Organizational procedures for quality verification in diagnostic work foster trust-based methodological rigour and local validation. Methodological rigour underpins trust when AI emerges as an organizational means. Trained on accurate data, AI “never gets tired and never makes mistakes”, addressing interviewees’ concerns for variations in diagnosis quality over time [39]. “Verified data sets are crucial to provide valuable support as references or maps guiding the radiologist”. At the same time, the interviewees point out that a challenge of verifying data is that the algorithm learns from standardized datasets and therefore lacks the ability to adapt to local knowledge [42]. The second theme addressing quality verification serving trust in AI was the need for a local validation process sensitive to variations in modalities and work processes. Local demography requires datasets specific to that particular region or cohort and cater for differences between modalities, even if they come from the same manufacturer. It was suggested that human-machine learning was needed to deal with a potential bias from the data and how it influences radiological evaluation.
The results show that radiologists’ trust in AI depends on the experience that AI is compatible with other systems and practices in the organization, increasing their capacity and providing control [43]. Capacity means that data from different sources is shared and integrated into a coherent infrastructure that leverages the organization’s capacity to plan, distribute, follow-up, and evaluate on an organizational level. Data sharing is crucial both within organizational units, between different hospitals, nationwide, and internationally to gain capacity. Trust in AI emerges when a variegated range of data formats are integrated into existing modalities so that experts across organizational or functional boundaries can share and use data to collaborate efficiently and safely. Integrated data must be coherent to support the management of the healthcare organization. However, in some cases, legal requirements regarding e.g. patient journals, personal data, and professional secrecy complicate control and validation procedures by creating tension between efficiency and patient integrity. To make AI increase trust in capacity building, the organization must have control over data. Variegated data sources and work processes make comparisons difficult, potentially delimiting trust. Having control over the data is also essential for monitoring the dataset distribution shift; continuous learning of the AI system on new data may lead to gradual change in the predicted outcomes. The organization must ensure though that this shift does not occur due to the bias in the training data.
To summarize, based on inter-organizational compatibility, trust in AI emerges when standardized procedures to follow-up, manage, and evaluate are fair, legal, and secure.
This study comes with certain limitations that could constrain the generalizability of the findings in a different context. The interviewees were selected purposively, resulting in a selection bias, which limits the results to their perspectives only. Furthermore, we used an explorative approach and open coding to analyse the interviews instead of consolidated criteria. While this approach allows for a freer exploration of the topic, it also comes with a risk of biased answers and misunderstanding of the topic between the interviewers and interviewees.
Conclusions
Trust in AI in healthcare is a complex attitude that builds on various procedural and substantial demands. To define the requirements that promote trust in AI, trust can be approached as a leap of faith rather than absolute certainty, as the latter may not be achievable or even desirable in this context. The procedural and substantial demands for trust identified in this study are conditioned on aspects related to reliability, transparency, quality verification, and inter-organizational compatibility. Each of these aspects is further divided into specific conditions that must be fulfilled. Creating the conditions for trust to emerge requires the involvement of various stakeholders, who will have to compensate the problem’s inherent complexity by finding and promoting well-defined solutions.
Abbreviations
- ADM:
-
Automated decision-making
- AI:
-
Artificial intelligence
References
Jones C, Thornton J, Wyatt JC (2021) Enhancing trust in clinical decision support systems: a framework for developers. BMJ Health Care Inform 28:e100247
Samek W, Wiegand T, Müller K-R (2017) Explainable artificial intelligence: understanding, visualizing and interpreting deep learning models. arXiv:170808296
Sahiner B, Pezeshk A, Hadjiiski LM et al (2019) Deep learning in medical imaging and radiation therapy. Med Phy 46:e1–e36
Yang L, Ene IC, Arabi Belaghi R, Koff D, Stein N, Santaguida P (2021) Stakeholders’ perspectives on the future of artificial intelligence in radiology: a scoping review. Eur Radiol 32:1477–1495
Gille F, Jobin A, Ienca M (2020) What we talk about when we talk about trust: theory of trust for AI in healthcare. Intell-Based Med 1–2:100001
Fuhrman JD, Gorre N, Hu Q, Li H, El Naqa I, Giger ML (2022) A review of explainable and interpretable AI with applications in COVID-19 imaging. Med Phys 49:1–14
Hasani N, Morris MA, Rahmim A et al (2022) Trustworthy artificial intelligence in medical imaging. PET Clin 17:1–12
Heinrichs B, Eickhoff SB (2020) Your evidence? Machine learning algorithms for medical diagnosis and prediction. Human Brain Mapping 41:1435–1444
Zhang Z, Genc Y, Wang D, Ahsen ME, Fan X (2021) Effect of AI explanations on human perceptions of patient-facing AI-powered healthcare systems. J Med Syst 45:64
Roscher R, Bohn B, Duarte MF, Garcke J (2020) Explainable machine learning for scientific insights and discoveries. IEEE Access 8:42200–42216
Linardatos P, Papastefanopoulos V, Kotsiantis S (2020) Explainable AI: a review of machine learning interpretability methods. Entropy (Basel) 23(1):18
Luhmann N (1979) Trust and power: two works, 1st edn. Wiley, Chichester, New York
Meyer S, Ward P, Coveney J, Rogers W (2008) Trust in the health system: an analysis and extension of the social theories of Giddens and Luhmann. Health Soc Rev 17:177–186
Beck U, Giddens A, Lash S (1994) Risk, trust, reflexivity. In: Reflexive modernization: politics, tradition and aesthetics in the modern social order. 1st edn. Stanford University Press
LaRosa E, Danks D (2018) Impacts on trust of healthcare AI. In: Proceedings of the 2018 AAAI/ACM Conference on AI, Ethics, and Society. Association for Computing Machinery, New York, NY, USA
Mayer RC, Davis JH, Schoorman FD (1995) An integrative model of organizational trust. Acad Manage Rev 20:709–734
Nikolova N, Möllering G, Reihlen M (2015) Trusting as a ‘Leap of Faith’: trust-building practices in client–consultant relationships. Scand J Manag 31:232–245
Ryan M (2020) In AI we trust: ethics, artificial intelligence, and reliability. Sci Eng Ethics 26:2749–2767
Strohm L, Hehakaya C, Ranschaert ER, Boon WP, Moors EH (2020) Implementation of artificial intelligence (AI) applications in radiology: hindering and facilitating factors. Eur Radiol 30:5525–5532
Park CJ, Yi PH, Siegel EL (2021) Medical student perspectives on the impact of artificial intelligence on the practice of medicine. Curr Probl Diagn Radiol 50:614–619
Aung YYM, Wong DCS, Ting DSW (2021) The promise of artificial intelligence: a review of the opportunities and challenges of artificial intelligence in healthcare. Br Med Bull 139:4–15
Pinto dos Santos D, Giese D, Brodehl S et al (2019) Medical students’ attitude towards artificial intelligence: a multicentre survey. Eur Radiol 29:1640–1646
Gryska E, Schneiderman J, Björkman-Burtscher I, Heckemann RA (2021) Automatic brain lesion segmentation on standard magnetic resonance images: a scoping review. BMJ Open 11:e042660
Bryman A (2012) Social research methods, 4th edn. Oxford University Press, Oxford, New York
Flyvbjerg B (2006) Five misunderstandings about case-study research. Qual Inq 12:219–245
Kvale S (2008) Doing interviews: qualitative research kit, 2nd edn. SAGE Publications, London
Gubrium JF, Holstein JA, Marvasti AB, McKinney KD (2012) The SAGE handbook of interview research: the complexity of the craft 2nd edn. SAGE Publications
Noordegraaf M (2020) Protective or connective professionalism? How connected professionals can (still) act as autonomous and authoritative experts. J Prof Organ 7:205–223
Evetts J (2011) A new professionalism? Challenges and opportunities. Curr Soc 59:406–422
Magrabi F, Ammenwerth E, McNair JB et al (2019) Artificial intelligence in clinical decision support: challenges for evaluating AI and practical implications. Yearb Med Inform 28:128–134
Svensson AM, Jotterand F (2022) Doctor Ex Machina: a critical assessment of the use of artificial intelligence in health care. J Med Philos 47:155–178
Bygstad B, Øvrelid E, Lie T, Bergquist M (2020) Developing and organizing an analytics capability for patient flow in a general hospital. Inf Syst Front 22:353–364
Bygstad B, Bergquist M (2018) Horizontal affordances for patient centred care in hospitals. Hawaii International Conference on System Sciences, HICSS-51, Waikoloa Village, Hawaii, USA, pp 3170–3179
Galozy A (2021) Data-driven personalized healthcare: towards personalized interventions via reinforcement learning for Mobile Health. Halmstad University Press, PhD diss.
Calisto FM, Nunes N, Nascimento JC (2022) Modeling adoption of intelligent agents in medical imaging. Int J Human-Comput Stud 168:102922
Harisinghani MG, O’Shea A, Weissleder R (2019) Advances in clinical MRI technology. Sci Trans Med 11:eaba2591
Coppola F, Faggioni L, Regge D et al (2021) Artificial intelligence: radiologists’ expectations and opinions gleaned from a nationwide online survey. Radiol Med 126:63–71
Schwartz JM, George M, Rossetti SC et al (2022) Factors influencing clinician trust in predictive clinical decision support systems for in-hospital deterioration: qualitative descriptive study. JMIR Hum Factors 9:e33960
Hemmer P, Schemmer M, Riefle L, et al (2022) Factors that influence the adoption of human-AI collaboration in clinical decision-making. arXiv:2204.09082
Reddy S (2022) Explainability and artificial intelligence in medicine. Lancet Digit Health 4(4):e214–e215
Amann J, Blasimme A, Vayena E, Frey D, Madai VI (2020) Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med Inform Decis Mak 20:310
Romero-Brufau S, Wyatt KD, Boyum P, Mickelson M, Moore M, Cognetta-Rieke C (2020) A lesson in implementation: a pre-post study of providers’ experience with artificial intelligence-based clinical decision support. Int J Med Inform 137:104072
Matthiesen S, Diederichsen SZ, Hansen MKH et al (2021) Clinician preimplementation perspectives of a decision-support tool for the prediction of cardiac arrhythmia based on machine learning: near-live feasibility and qualitative study. JMIR Hum Factors 8:e26964
Funding
Open access funding provided by University of Gothenburg. This study was funded under the agreement on medical education and research (ALFGBG 925851, ALFGBG 966177) and Region Västra Götaland (Innovationsfonden 940050).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Isabella M. Björkman-Burtscher.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Not applicable—Written informed consent was not required for this study as no patient or personal data were collected.
Ethical approval
Not applicable—The conducted research does not require ethical approval according to applicable national/Swedish law.
Methodology
• exploratory study
• performed at multiple institutions
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bergquist, M., Rolandsson, B., Gryska, E. et al. Trust and stakeholder perspectives on the implementation of AI tools in clinical radiology. Eur Radiol 34, 338–347 (2024). https://doi.org/10.1007/s00330-023-09967-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09967-5