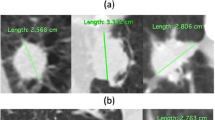
Abstract
Objectives
To evaluate the performance of automatic deep learning (DL) algorithm for size, mass, and volume measurements in predicting prognosis of lung adenocarcinoma (LUAD) and compared with manual measurements.
Methods
A total of 542 patients with clinical stage 0-I peripheral LUAD and with preoperative CT data of 1-mm slice thickness were included. Maximal solid size on axial image (MSSA) was evaluated by two chest radiologists. MSSA, volume of solid component (SV), and mass of solid component (SM) were evaluated by DL. Consolidation-to-tumor ratios (CTRs) were calculated. For ground glass nodules (GGNs), solid parts were extracted with different density level thresholds. The prognosis prediction efficacy of DL was compared with that of manual measurements. Multivariate Cox proportional hazards model was used to find independent risk factors.
Results
The prognosis prediction efficacy of T-staging (TS) measured by radiologists was inferior to that of DL. For GGNs, MSSA-based CTR measured by radiologists (RMSSA%) could not stratify RFS and OS risk, whereas measured by DL using 0HU (2D−AIMSSA0HU%) could by using different cutoffs. SM and SV measured by DL using 0 HU (AISM0HU% and AISV0HU%) could effectively stratify the survival risk regardless of different cutoffs and were superior to 2D−AIMSSA0HU%. AISM0HU% and AISV0HU% were independent risk factors.
Conclusion
DL algorithm can replace human for more accurate T-staging of LUAD. For GGNs, 2D−AIMSSA0HU% could predict prognosis rather than RMSSA%. The prediction efficacy of AISM0HU% and AISV0HU% was more accurate than of 2D−AIMSSA0HU% and both were independent risk factors.
Clinical relevance statement
Deep learning algorithm could replace human for size measurements and could better stratify prognosis than manual measurements in patients with lung adenocarcinoma.
Key Points
• Deep learning (DL) algorithm could replace human for size measurements and could better stratify prognosis than manual measurements in patients with lung adenocarcinoma (LUAD).
• For GGNs, maximal solid size on axial image (MSSA)–based consolidation-to-tumor ratio (CTR) measured by DL using 0 HU could stratify survival risk than that measured by radiologists.
• The prediction efficacy of mass- and volume-based CTRs measured by DL using 0 HU was more accurate than of MSSA-based CTR and both were independent risk factors.





Similar content being viewed by others
Abbreviations
- 2D − AIMSSA0HU :
-
MSSA evaluated by AI using 0 HU-2D project
- 2D − AIMSSA0HU%:
-
MSSA-to-tumor ratio evaluated by AI using 0HU-2D project
- AISM0HU%:
-
Mass of solid component (SM)-to-tumor ratio evaluated by AI using 0 HU
- AISV0HU%:
-
Volume of solid component (SV)-to-tumor ratio evaluated by AI using 0 HU
- AITS0HU :
-
T stage of the eighth edition staging (TS) evaluated by AI using 0 HU
- RMSSA:
-
Maximal solid size on axial image (MSSA) evaluated by radiologists
- RMSSA%:
-
MSSA-to-tumor ratio evaluated by radiologists
- RMTSA:
-
Maximal total size on axial image (MTSA) evaluated by radiologists
- RTS:
-
T stage of the eighth edition staging (TS) evaluated by radiologists
References
MacMahon H, Naidich DP, Goo JM et al (2017) Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 284(1):228–243
Travis WD, Asamura H, Bankier AA et al (2016) The IASLC Lung Cancer Staging Project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM Classification of Lung Cancer. J Thorac Oncol 11(8):1204–23
Rusch VW, Chansky K, Kindler HL et al (2016) The IASLC Mesothelioma Staging Project: proposals for the M descriptors and for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Mesothelioma. J Thorac Oncol 11(12):2112–2119
Travis WD, Brambilla E, Noguchi M et al (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6(2):244–285
Hattori A, Hirayama S, Matsunaga T et al (2019) Distinct clinicopathologic characteristics and prognosis based on the presence of ground glass opacity component in clinical stage IA lung adenocarcinoma. J Thorac Oncol 14(2):265–275
Lee KH, Goo JM, Park SJ et al (2014) Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol 9(1):74–82
Kudo Y, Matsubayashi J, Saji H et al (2015) Association between high-resolution computed tomography findings and the IASLC/ATS/ERS classification of small lung adenocarcinomas in Japanese patients. Lung Cancer 90(1):47-54
Lederlin M, Puderbach M, Muley T et al (2013) Correlation of radio- and histomorphological pattern of pulmonary adenocarcinoma. Eur Respir J 41(4):943–951
Ye T, Deng L, Wang S et al (2019) Lung adenocarcinomas manifesting as radiological part-solid nodules define a special clinical subtype. J Thorac Oncol 14(4):617–627
Soh J, Toyooka S, Shintani Y et al (2022) Limited resection for stage IA radiologically invasive lung cancer: a real-world nationwide database study. Eur J Cardiothorac Surg 2022:62(1)
Saji H, Okada M, Tsuboi M et al (2022) Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399(10335):1607–1617
Tsutani Y, Suzuki K, Koike T et al (2019) High-risk factors for recurrence of stage I lung adenocarcinoma: follow-up data from JCOG0201. Ann Thorac Surg 108(5):1484–1490
Suzuki K, Saji H, Aokage K et al (2019) Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg 158(3):895–907
Suzuki K, Koike T, Asakawa T et al (2011) A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 6(4):751–756
Matsunaga T, Suzuki K, Hattori A et al (2013) Lung cancer with scattered consolidation: detection of new independent radiological category of peripheral lung cancer on thin-section computed tomography. Interact Cardiovasc Thorac Surg 16(4):445–449
Kawaguchi Y, Nakao M, Omura K et al (2020) The utility of three-dimensional computed tomography for prediction of tumor invasiveness in clinical stage IA lung adenocarcinoma. J Thorac Dis 12(12):7218–7226
Hamanaka K, Takayama H, Koyama T et al (2019) Interobserver size measurement variability in part-solid lung adenocarcinoma using pre-operative computed tomography. J Thorac Dis 11(7):2924–2931
Scholten ET, Jacobs C, van Ginneken B et al (2015) Detection and quantification of the solid component in pulmonary subsolid nodules by semiautomatic segmentation. Eur Radiol 25(2):488-96
Cohen JG, Goo JM, Yoo RE et al Software performance in segmenting ground-glass and solid components of subsolid nodules in pulmonary adenocarcinomas. Eur Radiol 26(12):4465-74
Garzelli L, Goo JM, Ahn SY et al (2018) Improving the prediction of lung adenocarcinoma invasive component on CT: value of a vessel removal algorithm during software segmentation of subsolid nodules. Eur J Radiol 100:58–65
Shimomura M, Iwasaki M, Ishihara S, Inoue M (2022) Volume-based consolidation-to-tumor ratio is a useful predictor for postoperative upstaging in stage I and II lung adenocarcinomas. Thorac Cardiovasc Surg 70(3):265–272
Kamiya S, Iwano S, Umakoshi H et al (2018) Computer-aided volumetry of part-solid lung cancers by using CT: solid component size predicts prognosis. Radiology 287(3):1030–1040
Ahn Y, Lee SM, Noh HN et al (2021) Use of a commercially available deep learning algorithm to measure the solid portions of lung cancer manifesting as subsolid lesions at CT: comparisons with radiologists and invasive component size at pathologic examination. Radiology 299(1):202–210
Kawaguchi Y, Shimada Y, Murakami K et al (2022) Prognostic impact of artificial intelligence-based volumetric quantification of the solid part of the tumor in clinical stage 0-I adenocarcinoma. Lung Cancer 170:85–90
Zhao W, Yang J, Sun Y et al (2018) 3D Deep learning from CT scans predicts tumor invasiveness of subcentimeter pulmonary adenocarcinomas. Cancer Res 78(24):6881–6889
Kim H, Goo JM, Park CM (2018) Evaluation of T categories for pure ground-glass nodules with semi-automatic volumetry: is mass a better predictor of invasive part size than other volumetric parameters? Eur Radiol 28(10):4288–4295
Zhang S, Lin D, Yu Y et al (2022) Which will carry more weight when CTR > 0.5, solid component size, CTR, tumor size or SUVmax? Lung Cancer 164:14–22
Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K (2016) Neither maximum tumor size nor solid component size is prognostic in part-solid lung cancer: impact of tumor size should be applied exclusively to solid lung cancer. Ann Thorac Surg 102(2):407–415
Horeweg N, van Rosmalen J, Heuvelmans MA et al (2014) Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 15(12):1332-41
Takenaka T, Yamazaki K, Miura N, Mori R, Takeo S (2016) The prognostic impact of tumor volume in patients with clinical stage IA non-small cell lung cancer. J Thorac Oncol 11(7):1074–1080
Acknowledgements
Jian-Cheng Yang and Li Zhang provided technical support for this study.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82102109) and Shanghai Municipal Commission of Health and Family Planning Program (grant number 20184Y0037).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Qiong Li, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• prognostic study
• multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Chen, LL., Luo, YW. et al. Prognostic impact of deep learning–based quantification in clinical stage 0-I lung adenocarcinoma. Eur Radiol 33, 8542–8553 (2023). https://doi.org/10.1007/s00330-023-09845-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09845-0