Abstract
Objectives
To evaluate whether MRI-based T stage (TMRI), [18F]FDG PET/CT-based N (NPET/CT), and M stage (MPET/CT) are superior in NPC patients’ prognostic stratification based on long-term survival evidences, and whether TNM staging method involving TMRI + NPET/CT + MPET/CT could improve NPC patients’ prognostic stratification.
Methods
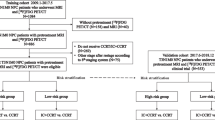
From April 2007 to December 2013, 1013 consecutive untreated NPC patients with complete imaging data were enrolled. All patients’ initial stages were repeated based on (1) the NCCN guideline recommended “TMRI + NMRI + MPET/CT” (“MMP”) staging method; (2) the traditional “TMRI + NMRI + Mconventional work-up (CWU)” (“MMC”) staging method; (3) the single-step “TPET/CT + NPET/CT + MPET/CT” (“PPP”) staging method; or (4) the “TMRI + NPET/CT + MPET/CT” (“MPP”) staging method recommended in present research. Survival curve, ROC curve, and net reclassification improvement (NRI) analysis were used to evaluate the prognosis predicting ability of different staging methods.
Results
[18F]FDG PET/CT performed worse on T stage (NRI = − 0.174, p < 0.001) but better on N (NRI = 0.135, p = 0.004) and M stage (NRI = 0.126, p = 0.001). The patients whose N stage upgraded by [18F]FDG PET/CT had worse survival (p = 0.011). The “TMRI + NPET/CT + MPET/CT” (“MPP”) method performed better on survival prediction when compared with “MMP” (NRI = 0.079, p = 0.007), “MMC” (NRI = 0.190, p < 0.001), or “PPP” method (NRI = 0.107, p < 0.001). The “TMRI + NPET/CT + MPET/CT” (“MPP”) method could reclassify patients’ TNM stage to a more appropriate stage. The improvement is significant in patients with more than 2.5-years follow-up according to the time-dependent NRI values.
Conclusions
The MRI is superior to [18F]FDG PET/CT in T stage, and [18F]FDG PET/CT is superior to CWU in N/M stage. The “TMRI + NPET/CT + MPET/CT” (“MPP”) staging method could significantly improve NPC patients’ long-term prognostic stratification.
Clinical relevance statement
The present research provided long-term follow-up evidence for benefits of MRI and [18F]FDG PET/CT in TNM staging for nasopharyngeal carcinoma, and proposes a new imaging procedure for TNM staging incorporating MRI-based T stage and [18F]FDG PET/CT-based N and M stage, which significantly improves long-term prognostic stratification for patients with NPC.
Key Points
• The long-term follow-up evidence of a large-scale cohort was provided to evaluate the advantages of MRI, [ 18 F]FDG PET/CT, and CWU in the TNM staging of nasopharyngeal carcinoma.
• A new imaging procedure for TNM stage of nasopharyngeal carcinoma was proposed.






Similar content being viewed by others
Abbreviations
- [18F]FDG:
-
[18F]Fluorodeoxyglucose
- “MMC” staging method:
-
The TNM staging method involving MRI based T and N stage and CWU-based M stage
- “MMP” staging method:
-
The TNM staging method involving MRI based T and N stage and [18F]FDG PET/CT-based M stage
- “MPP” staging method:
-
The TNM staging method involving MRI based T stage and [18F]FDG PET/CT-based N and M stage
- “PPP” staging method:
-
The TNM staging method involving [18F]FDG PET/CT-based T, N, and M stage
- 2DRT:
-
2-Dimensional radiotherapy
- AC:
-
Adjuvant chemotherapy
- AUC:
-
Area under the receiver-operating-characteristic curve
- CLN:
-
Cervical lymph node
- CT:
-
Computed tomography
- CTVs:
-
Clinical target volumes
- CWU:
-
Conventional workup
- DNA:
-
Deoxyribonucleic acid
- EBV:
-
Epstein-Barr virus
- FOV:
-
Field of view
- GTV:
-
Gross tumor volume
- Gy:
-
Gray
- HNSCC:
-
Head and neck squamous cell carcinoma
- IMRT:
-
Intensity-modulated radiation therapy
- IQR:
-
Interquartile range
- LN:
-
Lymph node
- MCWU :
-
M stage based on CWU imaging
- MPET/CT :
-
M stage based on [18F]FDG PET/CT imaging
- MRI:
-
Magnetic resonance imaging
- NACT:
-
Neoadjuvant chemotherapy
- NCCN:
-
National Comprehensive Cancer Network
- NMRI :
-
N stage based on MRI imaging
- NPC:
-
Nasopharyngeal carcinoma
- NPET/CT :
-
N stage based on [18F]FDG PET/CT imaging
- NRI:
-
The net reclassification improvement
- OS:
-
Overall survival
- PET/CT:
-
Positron emission tomography and computed tomography
- RI:
-
Reclassification improvement
- RLN:
-
Retropharyngeal lymph node
- RT:
-
Radiation therapy
- TMRI :
-
T stage based on MRI imaging
- TNM:
-
Tumor node metastasis
- TPET/CT :
-
T stage based on [18F]FDG PET/CT imaging
- UICC:
-
The Union for International Cancer Control
- WHO:
-
World Health Organization
References
Cao S-M, Simons MJ, Qian C-N (2011) The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30:114
Lee AW, Lin JC, Ng WT (2012) Current management of nasopharyngeal cancer. Semin Radiat Oncol 22:233–244
National Comprehensive Cancer Network. Head and Neck Cancers (Version 2.2022). Available via https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed 26 Apr 2022
Webber C, Gospodarowicz M, Sobin LH et al (2014) Improving the TNM classification: findings from a 10-year continuous literature review. Int J Cancer 135:371–378
Chen Y-P, Chan AT, Le Q-T, Blanchard P, Sun Y, Ma J (2019) Nasopharyngeal carcinoma. Lancet 394:64-80
Beyer T, Townsend DW, Brun T et al (2000) A combined PET/CT scanner for clinical oncology. J Nucl Med 41:1369–1379
Ng S-H, Yen T-C, Chang JT-C et al (2006) Prospective study of (18F) fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J Clin Oncol 24:4371–4376
Ng S-H, Chan S-C, Yen T-C et al (2009) Staging of untreated nasopharyngeal carcinoma with PET/CT: comparison with conventional imaging work-up. Eur J Nucl Med Mol Imaging 36:12
Kim JH, Choi KY, Lee S-H et al (2020) The value of CT, MRI, and PET-CT in detecting retropharyngeal lymph node metastasis of head and neck squamous cell carcinoma. BMC Med Imaging 20:1–8
Lowe VJ, Duan F, Subramaniam RM et al (2019) Multicenter trial of [18F] fluorodeoxyglucose positron emission tomography/computed tomography staging of head and neck cancer and negative predictive value and surgical impact in the N0 neck: results from ACRIN 6685. J Clin Oncol 37:1704
Lonneux M, Hamoir M, Reychler H et al (2010) Positron emission tomography with [18F] fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J Clin Oncol 28:1190–1195
Troost EG, Schinagl DA, Bussink J, Oyen WJ, Kaanders JH (2010) Clinical evidence on PET–CT for radiation therapy planning in head and neck tumours. Radiother Oncol 96:328–334
Facey K, Bradbury I, Laking G, Payne E (2007) Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technology Assessment –Southampton 11:44
Hafidh MA, Lacy PD, Hughes JP, Duffy G, Timon CV (2006) Evaluation of the impact of addition of PET to CT and MR scanning in the staging of patients with head and neck carcinomas. Eur Arch Otorhinolaryngol 263:853
Comoretto M, Balestreri L, Borsatti E, Cimitan M, Franchin G, Lise M (2008) Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: comparison of MR imaging and FDG PET/CT. Radiology 249:203–211
Tang L-Q, Chen Q-Y, Fan W et al (2013) Prospective study of tailoring whole-body dual-modality [18F] fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 31:2861–2869
Liu F-Y, Chang JT, Wang H-M et al (2006) [18F] fluorodeoxyglucose positron emission tomography is more sensitive than skeletal scintigraphy for detecting bone metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 24:599–604
Chang JT-C, Chan S-C, Yen T-C et al (2005) Nasopharyngeal carcinoma staging by (18) F-fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys 62:501–507
Shanmugaratnam K, Sobin LH (1993) The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer 71:2689–2697
Kim M, Roh J-L, Kim J et al (2007) Utility of 18F-fluorodeoxyglucose positron emission tomography in the preoperative staging of squamous cell carcinoma of the oropharynx. Eur J Surg Oncol 33:633–638
Ng SH, Yen TC, Chang JT et al (2006) Prospective study of [18F]fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J Clin Oncol 24:4371-4376
Ryu IS, Roh J-L, Kim JS et al (2016) Impact of 18F-FDG PET/CT staging on management and prognostic stratification in head and neck squamous cell carcinoma: a prospective observational study. Eur J Cancer 63:88–96
Amin MB, Edge S, Greene F et al (2017) AJCC Cancer Staging Manual, 8th edn. Springer, New York
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 1977:159–174
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Measur 20:37–46
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363
Pencina MJ, D’Agostino RB, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27:157–172
Inker LA, Schmid CH, Tighiouart H et al (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367:20–29
Pischon T, Boeing H, Hoffmann K et al (2008) General and abdominal adiposity and risk of death in Europe. N Engl J Med 359:2105–2120
D’agostino RB, Vasan RS, Pencina MJ et al (2008) General cardiovascular risk profile for use in primary care. Circulation 117:743–753
Steyerberg EW, Vickers AJ, Cook NR et al (2010) Assessing the performance of prediction models: a framework for some traditional and novel measures. Epidemiology 21:128
Zethelius B, Berglund L, Sundström J et al (2008) Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 358:2107–2116
Nakamura A, Kaneko N, Villemagne VL et al (2018) High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 554:249
Lehmann N, Erbel R, Mahabadi AA et al (2018) Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the HNR study (Heinz Nixdorf Recall). Circulation 137:665–679
Koshy M, Paulino AC, Howell R, Schuster D, Halkar R, Davis LW (2005) F‐18 FDG PET‐CT fusion in radiotherapy treatment planning for head and neck cancer. Head Neck-J Sci Spec 27:494–502
Kitajima K, Nakamoto Y, Okizuka H et al (2008) Accuracy of whole-body FDG-PET/CT for detecting brain metastases from non-central nervous system tumors. Ann Nucl Med 22:595–602
Li Y, Jin G, Su D (2017) Comparison of gadolinium-enhanced MRI and 18FDG PET/PET-CT for the diagnosis of brain metastases in lung cancer patients: a meta-analysis of 5 prospective studies. Oncotarget 8:35743–35749
VanderWalde NA, Salloum RG, Liu T-L et al (2014) Positron emission tomography and stage migration in head and neck cancer. JAMA Otolaryngol Head Neck Surg 140:654–661
Zhang W, Chen Y, Chen L et al (2015) The clinical utility of plasma Epstein–Barr virus DNA assays in nasopharyngeal carcinoma: the dawn of a new era?: A systematic review and meta-analysis of 7836 cases. Medicine (Baltimore) 94:20
Chan S, Yeh C, Yen T et al (2018) Clinical utility of simultaneous whole-body F-FDG PET/MRI as a single-step imaging modality in the staging of primary nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging 45:1297–1308
Stolzmann P, Veit-Haibach P, Chuck N et al (2013) Detection rate, location, and size of pulmonary nodules in trimodality PET/CT-MR: comparison of low-dose CT and Dixon-based MR imaging. Invest Radiol 48:241–246
Funding
This work was supported by grants from the National Key R&D Program of China (SQ2022YFC2500174), the National Natural Science Foundation of China (No. 32200651, 82203776, 82203125, 82222050, 82272739, 82272882, 82173287, 82073003, 82003267, 82002852), Guangdong Major Project of Basic and Applied Basic Research (HRB103), the Sci-Tech Project Foundation of Guangzhou City (202201011561), the Sun Yat-sen University Clinical Research 5010 Program (No. 201315, 2015021, 2017010, 2019023), Innovative Research Team of High-level Local Universities in Shanghai (SSMU-ZLCX20180500), the Special Support Plan of Guangdong Province (No. 2014TX01R145), the Natural Science Foundation of Guangdong Province (No.2017A030312003, No.2018A0303131004), the Natural Science Foundation of Guangdong Province for Distinguished Young Scholar(No.2018B030306001), Postdoctoral Innovative Talent Support Program (BX20220361), the Sci-Tech Project Foundation of Guangdong Province (No. 2014A020212103), the Health and Medical Collaborative Innovation Project of Guangzhou City (No. 201400000001, No.201803040003), Pearl River S&T Nova Program of Guangzhou (No. 201806010135), the Planned Science and Technology Project of Guangdong Province (2019B020230002), the National Science and Technology Pillar Program during the Twelfth Five-year Plan Period (No. 2014BAI09B10), Key Youth Teacher Cultivating Program of Sun Yat-sen University (20ykzd24), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Lin-Quan Tang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, HJ., Sun, XS., Zhang, X. et al. Head and neck MRI-based T stage and [18F]FDG PET/CT-based N/M stage improved prognostic stratification in primary nasopharyngeal carcinoma. Eur Radiol 33, 7952–7966 (2023). https://doi.org/10.1007/s00330-023-09815-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09815-6