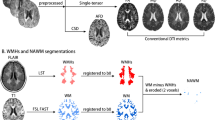
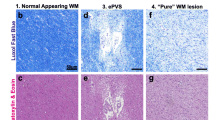
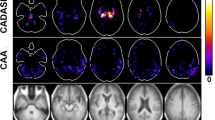
Abstract
Objectives
Venous pathology could contribute to the development of parenchymal lesions in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). We aim to identify presumed periventricular venous infarction (PPVI) in CADASIL and analyze the associations between PPVI, white matter edema, and microstructural integrity within white matter hyperintensities (WMHs) regions.
Methods
We included forty-nine patients with CADASIL from a prospectively enrolled cohort. PPVI was identified according to previously established MRI criteria. White matter edema was evaluated using the free water (FW) index derived from diffusion tensor imaging (DTI), and microstructural integrity was evaluated using FW-corrected DTI parameters. We compared the mean FW values and regional volumes with different levels of FW (ranging from 0.3 to 0.8) in WMHs regions between the PPVI and non-PPVI groups. We used intracranial volume to normalize each volume. We also analyzed the association between FW and microstructural integrity in fiber tracts connected with PPVI.
Results
We found 16 PPVIs in 10 of 49 CADASIL patients (20.4%). The PPVI group had larger WMHs volume (0.068 versus 0.046, p = 0.036) and higher FW in WMHs (0.55 versus 0.52, p = 0.032) than the non-PPVI group. Larger areas with high FW content were also found in the PPVI group (threshold: 0.7, 0.47 versus 0.37, p = 0.015; threshold: 0.8, 0.33 versus 0.25, p = 0.003). Furthermore, higher FW correlated with decreased microstructural integrity (p = 0.009) in fiber tracts connected with PPVI.
Conclusions
PPVI was associated with increased FW content and white matter degeneration in CADASIL patients.
Clinical relevance statement
PPVI is an important factor related with WMHs, and therefore, preventing the occurrence of PPVI would be beneficial for patients with CADASIL.
Key Points
•Presumed periventricular venous infarction is important and occurs in about 20% of patients with CADASIL.
•Presumed periventricular venous infarction was associated with increased free water content in the regions of white matter hyperintensities.
•Free water correlated with microstructural degenerations in white matter tracts connected with the presumed periventricular venous infarction.





Similar content being viewed by others
Abbreviations
- ANTs:
-
Advanced Normalization Tools
- CADASIL:
-
Cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- CSVD:
-
Cerebral small vessel diseases
- FCI:
-
Fiber pathways connecting the PPVI
- FW:
-
Free water
- ICV:
-
Intracranial volumes
- ISF:
-
Interstitial fluid
- MNI:
-
Montreal Neurological Institute
- PPVI:
-
Presumed periventricular venous infarction
- PVI:
-
Periventricular venous infarction
References
Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser M-G (2009) CADASIL. Lancet Neurol 8:643–653
Bordes C, Sargurupremraj M, Mishra A, Debette S (2022) Genetics of common cerebral small vessel disease. Nat Rev Neurol 18:84–101
Di Donato I, Bianchi S, De Stefano N et al (2017) Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med 15:41
Campbell BC, De Silva DA, Macleod MR et al (2019) Ischaemic stroke. Nat Rev Dis Primers 5:1–22
Keith J, Gao F-q, Noor R et al (2017) Collagenosis of the deep medullary veins: an underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction? J Neuropathol Exp Neurol 76:299–312
Pettersen JA, Keith J, Gao F, Spence JD, Black SE (2017) CADASIL accelerated by acute hypotension: arterial and venous contribution to leukoaraiosis. Neurology 88:1077–1080
De Guio F, Vignaud A, Ropele S et al (2014) Loss of venous integrity in cerebral small vessel disease: a 7-T MRI study in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Stroke 45:2124–2126
Rafalowska J, Fidzianska A, Dziewulska D, Podlecka A, Szpak GM, Kwiecinski H (2004) CADASIL or CADVaSIL? Neuropathology 24:16–20
Beggs CB (2013) Venous hemodynamics in neurological disorders: an analytical review with hydrodynamic analysis. BMC Med 11:1–17
Hu J, Shen Y, Fahmy LM et al (2023) The role of the parenchymal vascular system in cerebrospinal fluid tracer clearance. Eur Radiol 33:656–665
Bakker EN, Bacskai BJ, Arbel-Ornath M et al (2016) Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol 36:181–194
Geer CP, Grossman SA (1997) Interstitial fluid flow along white matter tracts: a potentially important mechanism for the dissemination of primary brain tumors. J Neurooncol 32:193–201
Schaller B, Graf R (2004) Cerebral venous infarction: the pathophysiological concept. Cerebrovasc Dis 18:179–188
Rasmussen MK, Mestre H, Nedergaard M (2022) Fluid transport in the brain. Physiol Rev 102:1025–1151
Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S (2015) What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 4:e001140
Duering M, Finsterwalder S, Baykara E et al (2018) Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers Dement 14:764–774
Yu X, Yin X, Hong H et al (2021) Increased extracellular fluid is associated with white matter fiber degeneration in CADASIL: in vivo evidence from diffusion magnetic resonance imaging. Fluids Barriers CNS 18:29
Yin X, Zhou Y, Yan S, Lou M (2019) Effects of cerebral blood flow and white matter integrity on cognition in CADASIL patients. Front Psych 9:741
Kirton A, DeVeber G, Pontigon AM, Macgregor D, Shroff M (2008) Presumed perinatal ischemic stroke: vascular classification predicts outcomes. Ann Neurol 63:436–443
Kirton A, Shroff M, Pontigon A-M, deVeber G (2010) Risk factors and presentations of periventricular venous infarction vs arterial presumed perinatal ischemic stroke. Arch Neurol 67:842–848
Yao M, Hervé D, Jouvent E et al (2014) Dilated perivascular spaces in small-vessel disease: a study in CADASIL. Cerebrovasc Dis 37:155–163
Eckstein K, Bachrata B, Hangel G et al (2021) Improved susceptibility weighted imaging at ultra-high field using bipolar multi-echo acquisition and optimized image processing: CLEAR-SWI. Neuroimage 237:118175
Pacella V, Foulon C, Jenkinson PM et al (2019) Anosognosia for hemiplegia as a tripartite disconnection syndrome. Elife 8:e46075
Schmidt P (2017) Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging. Ludwig-Maximilians-Universität München. Available via https://edoc.ub.uni-muenchen.de/20373/
Egger C, Opfer R, Wang C et al (2017) MRI FLAIR lesion segmentation in multiple sclerosis: does automated segmentation hold up with manual annotation? Neuroimage Clin 13: 264–270
Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y (2009) Free water elimination and mapping from diffusion MRI. Magn Reson Med 62:717–730
Wardlaw JM, Smith EE, Biessels GJ et al (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838
Schrag M, McAuley G, Pomakian J et al (2010) Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol 119:291–302
Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A (2019) Moving beyond P values: data analysis with estimation graphics. Nat Methods 16:565–566
Duering M, Csanadi E, Gesierich B et al (2013) Incident lacunes preferentially localize to the edge of white matter hyperintensities: insights into the pathophysiology of cerebral small vessel disease. Brain 136:2717–2726
Fulop GA, Tarantini S, Yabluchanskiy A et al (2019) Role of age-related alterations of the cerebral venous circulation in the pathogenesis of vascular cognitive impairment. Am J Physiol Heart Circ Physiol 316:H1124–H1140
Zhang R, Huang P, Jiaerken Y et al (2021) Venous disruption affects white matter integrity through increased interstitial fluid in cerebral small vessel disease. J Cereb Blood Flow Metab 41:157–165
Silvis SM, De Sousa DA, Ferro JM, Coutinho JM (2017) Cerebral venous thrombosis. Nat Rev Neurol 13:555–565
Manini A, Pantoni L (2021) CADASIL from bench to bedside: disease models and novel therapeutic approaches. Mol Neurobiol 58:2558–2573
Hartmann DA, Hyacinth HI, Liao FF, Shih AY (2018) Does pathology of small venules contribute to cerebral microinfarcts and dementia? J Neurochem 144:517–526
Funding
This study has received funding by the National Natural Science Foundation of China (No. 81901706, No. 82101984, No. 81901319, and No. 81571654), and the Natural Science Foundation of Zhejiang Province (Grant No. LSZ19H180001 and LQ20H180015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantors of this publication are Minming Zhang and Peiyu Huang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
•prospective
•cross-sectional study
•performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinfeng Yu and Xinzhen Yin contributed equally.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, X., Yin, X., Hong, H. et al. Presumed periventricular venous infarction on magnetic resonance imaging and its association with increased white matter edema in CADASIL. Eur Radiol 33, 8057–8066 (2023). https://doi.org/10.1007/s00330-023-09744-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09744-4