Abstract
Objectives
To characterize the structural plasticity of the contralesional hippocampus and its subfields in patients with unilateral glioma.
Methods
3D T1-weighted MRI images were collected from 55 patients with tumors infiltrating the left (HipL, n = 27) or right (HipR, n = 28) hippocampus, along with 30 age- and sex-matched healthy controls (HC). Gray matter volume differences of the contralesional hippocampal regions and three control regions (superior frontal gyrus, caudate nucleus, and superior occipital gyrus) were evaluated using voxel-based morphometry (VBM) analyses. Volumetric differences in the hippocampus and its subregional volume were measured using the FreeSurfer software.
Results
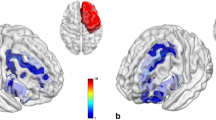
Compared with HC, patients with unilateral hippocampal glioma exhibited significantly larger gray matter volume in the contralesional hippocampus and parahippocampal regions (cluster = 571 voxels for HipL; cluster 1 = 538 voxels and cluster 2 = 88 voxels for HipR; family-wise error corrected p < 0.05). No significant alterations were found in control regions. Volumetric analyses showed the same trend in the contralesional hippocampal subregions for both patient groups, including the CA1 head, CA3 head, hippocampus amygdala transition area (HATA), fimbria, and the granule cell molecular layer of the dentate gyrus head (GC-ML-DG head). Notably, the differences of the contralesional HATA (HipL: η2 = 0.418, corrected p = 0.002; HipR: η2 = 0.313, corrected p = 0.052) and fimbria (HipL: η2 = 0.450, corrected p < 0.001; HipR: η2 = 0.358, corrected p = 0.012) still held after the Bonferroni correction.
Conclusions
Our findings provide evidence for macrostructural plasticity of the contralateral hippocampus in patients with unilateral hippocampal glioma. Specifically, HATA and fimbria exhibit great potential in this process.
Key Points
• Glioma infiltration of the hippocampal regions induces a significant increase in gray matter volume on the contralateral side.
• Specifically, the HATA and fimbria regions exhibit favorable plastic potential in the process of lesion-induced structural remolding.



Similar content being viewed by others
Abbreviations
- CA:
-
Cornu ammonis area
- DG:
-
Dentate gyrus
- GC-ML-DG:
-
The granule cell molecular layer of the dentate gyrus
- GM:
-
Gray matter
- HATA:
-
Hippocampus amygdala transition area
- HC:
-
Healthy controls
- HipL/R:
-
Patients with tumors infiltrating the left/right hippocampus
- IDH:
-
Isocitrate dehydrogenase
- LGG:
-
Lower-grade glioma
- MANCOVA:
-
Multivariate analysis of covariance
- MNI:
-
Montreal Neurological Institute
- ROI:
-
Region of interest
- TFCE-FWE:
-
Threshold-free cluster enhancement family-wise error
- TIV:
-
Total intracranial volume
- VBG:
-
Virtual brain grafting
- VBM:
-
Voxel-based morphometry
References
Cramer SC, Sur M, Dobkin BH et al (2011) Harnessing neuroplasticity for clinical applications. Brain 134(Pt 6):1591–1609
Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J et al (2019) Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med 25(4):554–560
Bliss T, Schoepfer R (2004) Neuroscience. Controlling the ups and downs of synaptic strength. Science 304(5673):973–4
Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC (2006) Storage of spatial information by the maintenance mechanism of LTP. Science 313(5790):1141–1144
Laitinen T, Sierra A, Pitkanen A, Grohn O (2010) Diffusion tensor MRI of axonal plasticity in the rat hippocampus. Neuroimage 51(2):521–530
Sierra A, Grohn O, Pitkanen A (2015) Imaging microstructural damage and plasticity in the hippocampus during epileptogenesis. Neuroscience 309:162–172
Knierim JJ (2015) The hippocampus. Curr Biol 25(23):R1116–R1121
Trimper JB, Galloway CR, Jones AC, Mandi K, Manns JR (2017) Gamma oscillations in rat hippocampal subregions dentate gyrus, CA3, CA1, and subiculum underlie associative memory encoding. Cell Rep 21(9):2419–2432
Hunsaker MR, Kesner RP (2008) Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus 18(9):955–964
Backus AR, Bosch SE, Ekman M, Grabovetsky AV, Doeller CF (2016) Mnemonic convergence in the human hippocampus. Nat Commun 7:11991
Gu Y, Janoschka S, Ge S (2013) Neurogenesis and hippocampal plasticity in adult brain. Curr Top Behav Neurosci 15:31–48
Leuner B, Gould E (2010) Structural plasticity and hippocampal function. Annu Rev Psychol 61:111–40, C1–3
Theodore WH, Bhatia S, Hatta J et al (1999) Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology 52(1):132–136
Pini L, Pievani M, Bocchetta M et al (2016) Brain atrophy in Alzheimer’s disease and aging. Ageing Res Rev 30:25–48
Herbet G, Maheu M, Costi E, Lafargue G, Duffau H (2016) Mapping neuroplastic potential in brain-damaged patients. Brain 139(Pt 3):829–844
Desmurget M, Bonnetblanc F, Duffau H (2007) Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain 130(Pt 4):898–914
Almairac F, Duffau H, Herbet G (2018) Contralesional macrostructural plasticity of the insular cortex in patients with glioma: a VBM study. Neurology 91(20):e1902–e1908
Liu D, Chen J, Hu X et al (2020) Contralesional homotopic functional plasticity in patients with temporal glioma. J Neurosurg 2020:1–9
Sanson M, Marie Y, Paris S et al (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27(25):4150–4154
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38(1):95–113
Ridgway GR, Litvak V, Flandin G, Friston KJ, Penny WD (2012) The problem of low variance voxels in statistical parametric mapping; a new hat avoids a ‘haircut.’ Neuroimage 59(3):2131–2141
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19(3):1233–1239
Radwan AM, Emsell L, Blommaert J et al (2021) Virtual brain grafting: enabling whole brain parcellation in the presence of large lesions. Neuroimage 229:117731
Avants BB, Tustison NJ, Song G et al (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54(3):2033–2044
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62(2):782–90
Tournier JD, Smith R, Raffelt D et al (2019) MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202:116137
Fischl B (2012) FreeSurfer Neuroimage 62(2):774–781
Brown EM, Pierce ME, Clark DC et al (2020) Test-retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. Neuroimage 210:116563
Iglesias JE, Augustinack JC, Nguyen K et al (2015) A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115:117–137
Yan CG, Wang XD, Zuo XN, Zang YF (2016) DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14(3):339–351
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44(1):83–98
May A, Hajak G, Ganssbauer S et al (2007) Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex 17(1):205–210
Bartsch T, Wulff P (2015) The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience 309:1–16
Weerasinghe-Mudiyanselage PDE, Ang MJ, Kang S, Kim JS, Moon C (2022) Structural plasticity of the hippocampus in neurodegenerative diseases. Int J Mol Sci 23(6):3349
Cembrowski MS, Spruston N (2019) Heterogeneity within classical cell types is the rule: lessons from hippocampal pyramidal neurons. Nat Rev Neurosci 20(4):193–204
Hsu D (2007) The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res 163:601–613
van Strien NM, Cappaert NL, Witter MP (2009) The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci 10(4):272–282
Nakao K, Matsuyama K, Matsuki N, Ikegaya Y (2004) Amygdala stimulation modulates hippocampal synaptic plasticity. Proc Natl Acad Sci U S A 101(39):14270–14275
Roesler R, Parent MB, LaLumiere RT, McIntyre CK (2021) Amygdala-hippocampal interactions in synaptic plasticity and memory formation. Neurobiol Learn Mem 184:107490
Saunders RC, Aggleton JP (2007) Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus 17(5):396–411
Yeo SS, Jang SH (2013) Neural reorganization following bilateral injury of the fornix crus in a patient with traumatic brain injury. J Rehabil Med 45(6):595–598
Zhang L, Hu X, Lu L et al (2019) Abnormalities of hippocampal shape and subfield volumes in medication-free patients with obsessive-compulsive disorder. Hum Brain Mapp 40(14):4105–4113
Giuliano A, Donatelli G, Cosottini M et al (2017) Hippocampal subfields at ultra high field MRI: an overview of segmentation and measurement methods. Hippocampus 27(5):481–494
Acknowledgements
The authors thank Prof. Ahmed M. Radwan and his team for their open-source code base, as well as the healthy volunteers and patients for their participation.
Funding
This study has received funding from the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. SJCX21_0643), the Jiangsu Provincial Medical Innovation Team (No. CXTDA2017050), and Jiangsu Provincial Health Commission (M2021003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Hongyi Liu.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• case–control study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, D., Chen, J., Ge, H. et al. Structural plasticity of the contralesional hippocampus and its subfields in patients with glioma. Eur Radiol 33, 6107–6115 (2023). https://doi.org/10.1007/s00330-023-09582-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09582-4