Abstract
Objectives
To compare vascular attenuation (VA) of an experimental half iodine-load dual-layer spectral detector CT (SDCT) lower limb computed tomography angiography (CTA) with control (standard iodine-load conventional 120-kilovolt peak (kVp) CTA).
Methods
Ethical approval and consent were obtained. In this parallel RCT, CTA examinations were randomized into experimental or control. Patients received 0.7 vs 1.4 mL/kg of iohexol 350 mgI/mL in the experimental- vs the control group. Two experimental virtual monoenergetic image (VMI) series at 40 and 50 kiloelectron volts (keV) were reconstructed. Primary outcome: VA. Secondary outcomes: image noise (noise), contrast- and signal-to-noise ratio (CNR and SNR), and subjective examination quality (SEQ).
Results
A total of 106 vs 109 were randomized and 103 vs 108 were analyzed in the experimental vs, control groups, respectively. VA was higher on experimental 40 keV VMI than on control (p < 0.0001), but lower on 50 keV VMI (p < 0.022). Noise was higher on experimental 40 keV VMI than on control (p = 0.00022), but lower on 50 keV VMI (p = 0.0033). CNR and SNR were higher than the control on experimental 40 keV VMI (both p < 0.0001) and 50 keV (p = 0.0058 and p = 0.0023, respectively). SEQ was better on both VMIs in the experimental group than in the control (both p < 0.0001).
Conclusions
Half iodine-load SDCT lower limb CTA at 40 keV achieved higher VA than the control. CNR, SNR, noise, and SEQ were higher at 40 keV, while 50 keV showed lower noise.
Clinical relevance statement
Spectral detector CT with low-energy virtual monoenergetic imaging performed halved iodine contrast medium (CM) lower limb CT-angiography with sustained objective and subjective quality. This facilitates CM reduction, improvement of low CM-dosage examinations, and examination of patients with more severe kidney impairment.
Trial registration
Retrospectively registered 5 August 2022 at clinicaltrials.gov NCT05488899.
Key Points
• Contrast medium dosage may be halved in lower limb dual-energy CT angiography with virtual monoenergetic images at 40 keV, which may reduce contrast medium consumption in the face of a global shortage.
• Experimental half-iodine-load dual-energy CT angiography at 40 keV showed higher vascular attenuation, contrast-to-noise ratio, signal-to-noise ratio, and subjective examination quality than standard iodine-load conventional.
• Half-iodine dual-energy CT angiography protocols may allow us to reduce the risk of PC-AKI, examine patients with more severe kidney impairment, and provide higher quality examinations or salvage poor examinations when impaired kidney function limits the CM dose.
Similar content being viewed by others
Introduction
Peripheral artery disease (PAD) is an important cause of death and disability worldwide [1]. PAD is characterized by atherosclerotic stenosis or occlusion in peripheral arteries, usually in the lower limbs, and the prevalence increases significantly with age [1].
Computed tomography angiography (CTA) is considered the examination of choice in PAD due to its availability, non-invasive nature, fast image acquisition, cost efficiency, and high diagnostic accuracy [2,3,4]. Although the incidence is low, post-contrast acute kidney injury (PC-AKI) remains a risk with CTA, especially for patients with severely impaired kidney function [5,6,7]. Reducing the contrast medium (CM) dosage may lessen this risk, which is worthwhile since PAD is associated with impaired kidney function, and lower limb CTA usually requires a large CM volume [8,9,10,11].
Reduction of CM dosage in CT, based on the increasing attenuation of iodine as photon energies approach the k-edge at 33.2 keV, may be accomplished with low kilovolt peak (kVp) or dual energy monoenergetic reconstruction [12].
Since dual-energy CT (DECT) was first described in 1973, vendors have introduced different techniques [13]. Dual-layer spectral detector CT (SDCT) uses one x-ray tube and a two-layer detector with different sensitivity for the low- and high photon spectrum energies. SDCT always captures spectral information at 120/140 kVp, so imaging protocols need not be modified for dual-energy scanning. The conventional images from the two detector layers are of equal quality to conventional scanners [14, 15].
With DECT, virtual monoenergetic images (VMI) are widely used to increase iodine attenuation and image contrast, especially in vascular imaging. The lowest energy levels of 40 and 50 keV, roughly equivalent to 70 and 80 kVp on single energy scanners, are the most promising with potential reductions in CM of 60%and 40%, respectively [16, 17]. VMI may also improve examination quality or salvage examinations with poor enhancement [18]. Few studies have reported on VMI in lower limb CTA.
Imaging protocols with greatly reduced CM-dosages may only be applied if clinical feasibility is ascertained, and the examination quality is adequate, and preferably equal or improved compared to conventional imaging [15].
Our hypothesis was that we could achieve a higher vascular attenuation by a half iodine-load SDCT lower limb CTA at 40 or 50 keV than with standard iodine-load at 120 kVp, and our primary objective was to compare the VA of these acquisitions. Our secondary objectives were to assess and compare contrast-to-noise ratio (CNR), signal-to-noise ratio (SNR), image noise (noise), and subjective examination quality (SEQ) between the two groups.
Methods
Patient population
Between 28 January 2019 and 16 October 2020, patients referred for lower limb CTA at University Hospital were considered for enrolment in this single-centre, parallel, randomized controlled trial (RCT). The inclusion criteria were: estimated glomerular filtration rate (eGFR) > 30 mL/min/1.73 m2 and clinical suspicion of- or known lower limb PAD. The exclusion criteria were: contraindication to iodinated CM, age < 18 years, pregnancy, or critical ischemia.
The study was approved by the Regional Committee for Medical and Health Research Ethics of South East Norway (ref 2018/473) and the Data Protection Officer of the hospital. All participants gave written informed consent. The study was retrospectively registered on 5 August 2022 at ClinicalTrials.gov (NCT05488899).
Randomisation
Examinations were randomized to either the experimental or control group. The principal investigator performed randomisation with a 1:1 ratio using the RANDBETWEEN (1,2) function in Microsoft Excel (Microsoft Corporation) and sealed the randomisation codes in sequentially numbered envelopes. Enrolment and allocation were performed by radiographers in the CT lab. The patients and personnel assessing outcomes were blinded to the randomisation.
Imaging protocol
We used a bodyweight-adapted CM volume of iohexol 350 mgI/mL (Omnipaque 350, GE Healthcare). The control group received the standard iodine load of 1.4 mL/kg, while the experimental group received 0.7 mL/kg as the CM was diluted 1:1 with saline. This allowed identical injection rate and -time in both groups.
The CM was administered using a power injector (CT Exprès TM 4D, Bracco Injeneering SA), with a 50 mL saline flush. We used an automatic bolus tracker with a region of interest (ROI) in the abdominal aorta (AA), 120 Hounsfield units (HU) trigger point, and scan after 15 s. The maximum injected volume was limited to 130 mL, and the minimum to 60 mL.
Scans were performed supine, feet-first on a Philips IQon SDCT scanner (Philips Healthcare). The scan parameters were: tube voltage: 120 kVp, collimation: 64*0.625 mm, rotation time: 0.5 s, pitch: 1.171, matrix: 512*512, slice thickness: 0.9 mm, increment: 0.45 mm. Automatic tube current (DoseRight 3D-DOM, Philips Healthcare) was enabled, and the Dose Right Index was set at 21.
Conventional images were reconstructed with hybrid iterative reconstruction, iDose 4, level 3, and VMI in 40 and 50 keV with a spectral reconstruction algorithm: spectral B, denoising level 3 on an IntelliSpace Portal 9.0 workstation (Philips Healthcare). In the control group, measurements from the conventional images were analyzed. In the experimental group, measurements from the VMIs at 40 and 50 keV were analyzed. All images were assessed in the axial plane with a 1 mm slice thickness.
Objective examination quality
One radiographer performed the analysis of objective examination quality (OEQ).
ROIs were placed manually in the AA at the mid-point between the renal arteries and the aortic bifurcation and bilaterally in.
-
the common iliac arteries (CIA) at the mid-point between the aortic bifurcation and internal iliac artery,
-
the superficial femoral arteries (SFA) 10 cm below the branching of the deep femoral artery, and
-
the P3 segment of the popliteal artery (PA).
Each ROI was drawn as large as possible to assess as much of the arterial lumen as possible without including the arterial wall or plaques. ROIs were copied and pasted in the VMI series to ensure measurements from identical areas. We recorded VA and standard deviation (SD). Background attenuation was measured in the closest muscle. OEQ was defined as VA (primary outcome), noise, CNR, and SNR (secondary outcomes). Noise was defined as SD of VA
Subjective examination quality
SEQ (secondary outcome) was independently rated by two vascular interventional radiologists. SEQ was rated in the AA, CIA, SFA, PA, and calf arteries. In the case of the three calf arteries, the artery with the best demarcation of the lumen was rated. In cases of occlusion, SEQ was not assessed at that anatomical level.
A 4-point rating scale was used, as follows: 1: Excellent, 2: good, 3: adequate, 4: non-diagnostic, where ratings 1 through 3 were considered diagnostic quality (Fig. 1). If both radiologists rated SEQ as diagnostic quality, the mean of the two scores was used for statistical analysis. Scores of 1.5 and 2.5 were termed “very good” and “quite good” respectively.
Disagreements on whether SEQ was non-diagnostic were resolved by a referee, a third vascular interventional radiologist. The resolved rating was the median of the three ratings.
Radiation dose estimation
The dose length product (DLP) and volume CT dose index (CTDIvol) was recorded for each patient. The effective dose (ED) was calculated as follows: ED = DLP*k, using the conversion coefficient k = 0,0056 mSv/mGycm [19].
Sample size and statistical analysis
Sample size calculation, with a 0.05 level of significance (α) and power (β) 80%, assuming a mean difference of 0.5 points in SEQ with an SD of 1.25, resulted in a sample size of 98 in each group.
Data analysis was performed with Statistical Package for the Social Sciences version 28.0 (IBM) and R version 4.2.0 (The R Foundation).
Differences between experimental and control groups in patient demographics and scan characteristics were assessed by independent samples t-test, and sex by chi-squared test.
Comparisons of OEQ and SEQ were performed with the Wilcoxon rank sum test for unpaired data between the control group and each of the VMIs in the experimental group, and Wilcoxon signed rank test for paired data within the experimental group between VMI at 40 and 50 keV.
Cohen’s D was used as effect size on the log-transformed OEQ data since attenuation, CNR and SNR showed normal distribution and noise was gamma distributed on the log scale. For SEQ the rank-biserial coefficient was reported as effect size. p values were inflated by a factor of three to account for the multiplicity of testing. All tests were two-sided, and with a 0.05 level of significance.
The influence of anatomical level, age, sex, and RCT groups on OEQ and SEQ were investigated by multivariable generalized mixed models. Patient ID was included as a random effect allowing for different latent mean values per patient. The log-transformed VA, CNR, and SNR were modelled by Gaussian likelihoods, while the log-transformed noise was modelled by a gamma likelihood, and an ordinal likelihood was used to model the resolved median subjective rating. Reported effect sizes and standard errors are those of the regression coefficients, which are in units of the (log-transformed) response variable per the units of the covariate.
Results
Between 28 January 2019 and 16 October 2020 880, lower limb CTAs were performed. We intended to enrol 216 examinations. However, one randomisation envelope went missing (experimental), and 215 were enrolled. Figure 2 shows a flowchart of enrolment, exclusion, and randomisation. One examination randomized to control received the experimental examination by mistake but was analyzed by intention to treat, according to randomisation. There were four post-randomisation exclusions. One patient was excluded due to eGFR < 30 mL/min/1.73 m2. Two examinations were excluded because the spectral base image data were not stored. One examination was excluded because the acquisition only included the abdomen and pelvis and not the lower limbs. During the inclusion period, 13 patients were referred, enrolled, and randomized twice, and one patient three times. Hence, 211 examinations of 182 patients were included in the analysis.
Table 1 shows patient- and scan characteristics in the experimental and control groups.
Primary outcome
Table 2 shows effect sizes and p values of differences in VA, CNR, SNR, and noise between experimental and control.
VA, the primary outcome, was higher in the experimental group at 40 keV than in the control group (p < 0.0001). VA was higher in the control group compared to the experimental group at 50 keV (p = 0.022).
Descriptive statistics showed that VA was consistently higher in the experimental group at 40 keV than in the control group, and experimental at 50 keV across all anatomical levels (Table 3). From the common iliac arteries and further distally the attenuation decreased.
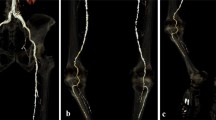
Table 4 shows generalized linear models predicting OEQ and SEQ using RCT group and patient factors. The effect of the experimental group remained clear on VMI at 40 keV (p < 0.0001), but not on VMI at 50 keV (p = 0.44) compared to the control. Figure 3 shows experimental and control examinations performed on the same patient.
Lower extremity CTA in the control group and in the experimental group with VMI at 50 and 40 keV. Axial images of a 76-year-old male who was referred to lower limb CTA on two occasions and examined with experimental and control protocols. ROIs placed in the abdominal aorta, common iliac artery, superficial femoral artery, and popliteal artery show attenuation and noise values, on conventional 120 kVp (control) and on VMI at 50 and 40 keV (experimental)
Secondary outcomes
Objective examination quality
Noise was lower in the experimental group at 50 keV than at 40 keV (p < 0.0001) and control (p = 0.00022), and higher at 40 keV than control (p = 0.0033) (Table 2). These effects remained significant in the multivariate analysis (Table 4).
At 40 keV, the experimental group showed higher CNR and SNR than at 50 keV (p < 0.0001) and control (p < 0.0001). The control showed lower CNR (p = 0.0058) and SNR (p = 0.0023) than the experimental at 50 keV (Table 2). In the multivariate analysis, only the effects on CNR and SNR between 40 keV and control remained clear (both p < 0.001) (Table 4). Table 3 shows OEQ for control and experimental across anatomical levels.
Subjective examination quality
The experimental group showed better SEQ at both 40 and 50 keV than the control (p < 0.0001) (Table 2). Within the experimental group, 40 keV showed better ratings than 50 keV (p < 0.0001). The difference in SEQ between the RCT groups was not significant in the multivariate analysis with an ordinal likelihood (Table 4). SEQ was most frequently rated unacceptable in the calf arteries, in 7.8%, 10.6%, and 11.1% in control, 40 keV and 50 keV, respectively (Fig. 4 and Table 5).
Discussion
In this study half iodine load SDCT showed higher VA and noise at 40 keV and lower VA and noise at 50 keV compared to the control, while CNR and SNR were higher at 40 keV.
Attenuation
We have shown that lower limb CTA with half iodine-load SDCT at 40 keV yielded higher VA across all anatomical levels than full iodine-load conventional CTA. The control showed higher VA than 50 keV, but the effect size was small and the p-value borderline significant, and the difference was not significant in the multivariate analysis. Reported optimal VA is approximately 350 HU, while 200–250 HU is clinically acceptable [20, 21]. In our study, the mean VA exceeded 350 HU in both RCT groups, at both VMIs and at all anatomical levels.
Image noise
Noise was lower at 50 keV than in the control, but the effect size was small. Noise was higher at 40 keV than both 50 keV and control with large and small effect sizes, respectively. This difference in effect size is partially due to the paired comparison between 40 and 50 keV. Thus, with different VMIs, DECT allows for both higher attenuation and lower noise, since both VMIs are available to the radiologist in clinical practice,
CNR and SNR
CNR and SNR were higher at 40 keV than at 50 keV and control with very small p values and small-to-medium effect sizes. The control showed the lowest CNR and SNR, and 50 keV was in between, with small p values and small-to-medium effect sizes. This indicates that the increase in attenuation at lower keV-values is larger than the increase in noise.
Subjective examination quality
SEQ was best at 40 keV with a small effect size compared to the control group and a large effect size compared to 50 keV, again partially due to paired testing. This might indicate that noise is less important to SEQ than attenuation, although this effect may be specific to CTA [22].
More examinations were rated as 3 (acceptable) or 4 (unacceptable) in the calf arteries than other anatomical levels. In the calf arteries, 7.8% were rated unacceptable in the control group compared to 10.6% and 11.1% in the intervention group at 40 and 50 keV, respectively. This might be due to blooming artefacts at low VMIs in calcified arteries. We did not test this difference statistically, but it may well be clinically significant as the calf and foot are often the areas of interest. The lower performance in distal vessels will have to be addressed by further protocol optimization.
Unfortunately, we cannot compare SEQ findings from the calf with objective measurements. We decided against measuring in the calf because it would be difficult to adequately place ROIs in very small and calcified vessels. Although some studies have made the same decision, others have made measurements of arterial attenuation in both calf and foot [23].
Acquisition techniques
Previous single energy CTA studies with 2/3rd CM dose have achieved higher attenuation, SNR, and CNR at 70 kVp but lower at 80 kVp, compared to full CM dose at 120 kVp, but no significant difference in subjective examination quality [24, 25].
A smaller study by Ren et al, which used a similar technique to ours, concluded that half of CM DECT yielded higher attenuation, CNR and SNR and lower noise at both 40 and 50 keV than standard CM at 120 kVp [26]. However, that study included only 40 patients and did not have sufficient statistical power to show significant differences neither in attenuation at 40 or 50 keV, nor noise at 40 keV. We included 211 examinations and were able to show significantly improved attenuation at 40 keV and significantly reduced noise at 50 keV.
Contrast administration techniques
CM administration technique is crucial for examination quality in CTA, and there are large variations in protocols. Ren et al used a fixed CM volume of 90 or 45 mL, while Almutairi et al used a weight-tailored CM dosage of 1.5 or 0.75 ml/kg [26, 27]. Both used 350 mgI/ml CM and their mean CM doses were similar to ours. In this study, we used a weight-tailored CM dosage. Individually tailored CM dosage may reduce iodine load and variation in enhancement compared to fixed dosage [28,29,30], and total body weight is the most commonly used strategy [31].
We replaced the reduced contrast volume in the experimental group with saline, keeping the injected volume, injection rate, CM concentration, and saline chaser unchanged, thus reducing the room for errors. Since we had no weight-based exclusion criteria, our results should be valid for the target population [30].
Clinical implications
Regular optimization of CT protocols is crucial to adapt CM injection protocols to evolving scanner technology, which requires less CM, without compromising examination quality. Although our study focused on lower limb CTA, our results might apply to other vascular CT examinations. We have shown that it is feasible in everyday clinical practice to halve the iodine load with SDCT CTA and sustain or even improve examination quality compared to 120 kVp conventional CT. This may allow us to reduce the risk of PC-AKI, examine patients with more severe kidney impairment and provide higher quality examinations or salvage poor examinations when impaired kidney function limits the CM dose [18].
In response to the ongoing global shortage of iodinated contrast media, DECT is one of the suggested approaches to reduce CM consumption. Although the number of DECT scanners are limited, and many facilities do not have access to DECT scanners, this might be expected to improve in the time to come.
Limitations
Our study was performed at a single centre and on one scanner only, limiting the external validity of our results for other DECT scanners. We limited our study to VMI reconstructions at 40 and 50 keV since previous studies identified these as the most promising [18, 26]. Including VMI at smaller intervals might have identified an optimal VMI between 40 and 50 keV. Since the experimental group showed less attenuation on conventional images, it was impossible to achieve complete blinding of the personnel assessing OEQ or SEQ to the RCT groups. Our measurements may have been influenced by clinical factors such as cardiovascular state, stenoses, or occlusions, but such influence is expected to be minimized by randomization. Although four patients were excluded after randomisation, we believe the risk of bias is small considering that the reasons for exclusions were not related to the outcomes, the large number of participants, and the strength of the observed effects.
Conclusion
Half iodine load SDCT lower limb CTA at 40 keV achieved higher vascular attenuation compared to conventional 120 kVp with standard iodine-load. CNR, SNR, noise, and subjective examination quality were higher at 40 keV while 50 keV showed lower noise levels.
Abbreviations
- AA:
-
Abdominal aorta
- CIA:
-
Common iliac arteries
- CM:
-
Contrast medium
- CNR:
-
Contrast-to-noise-ratio
- CTA:
-
Computed tomography angiography
- CTDIvol:
-
Volume CT dose index
- DECT:
-
Dual-energy CT
- DLP:
-
Dose length product
- ED:
-
Effective dose
- eGFR:
-
Estimated glomerular filtration rate
- HU:
-
Hounsfield units
- keV:
-
Kiloelectron volt
- kVp:
-
Kilovolt peak
- Noise:
-
Image noise
- OEQ:
-
Objective examination quality
- PA:
-
Popliteal artery
- PAD:
-
Peripheral artery disease
- PC-AKI:
-
Post-contrast acute kidney injury
- RCT:
-
Randomized controlled trial
- ROI:
-
Region of interest
- SDCT:
-
Dual-layer spectral detector CT
- SD:
-
Standard deviation
- SEQ:
-
Subjective examination quality
- SFA:
-
Superficial femoral arteries
- SNR:
-
Signal-to-noise-ratio
- VA:
-
Vascular attenuation
- VMI:
-
Virtual monoenergetic images
References
Song P, Rudan D, Zhu Y et al (2019) Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health 7:e1020–e1030
Ouwendijk R, de Vries M, Stijnen T et al (2008) Multicenter randomized controlled trial of the costs and effects of noninvasive diagnostic imaging in patients with peripheral arterial disease: the DIPAD trial. AJR Am J Roentgenol 190:1349–1357
Heijenbrok-Kal MH, Kock MC, Hunink MG (2007) Lower extremity arterial disease: multidetector CT angiography meta-analysis. Radiology 245:433–439
Kayhan A, Palabiyik F, Serinsoz S et al (2012) Multidetector CT angiography versus arterial duplex USG in diagnosis of mild lower extremity peripheral arterial disease: is multidetector CT a valuable screening tool? Eur J Radiol 81:542–546
Davenport MS, Perazella MA, Yee J et al (2020) Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology 294:660–668
van der Molen AJ, Reimer P, Dekkers IA et al (2018) Post-contrast acute kidney injury - Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors : Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 28:2845–2855
van der Molen AJ, Reimer P, Dekkers IA et al (2018) Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients : Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 28:2856–2869
Brown JR, Robb JF, Block CA et al (2010) Does safe dosing of iodinated contrast prevent contrast-induced acute kidney injury? Circ Cardiovasc Interv 3:346–350
Sarmento C, Pereira T, Maldonado J, Conde J (2013) Peripheral artery disease and kidney function in hypertensive patients. Arq Bras Cardiol 100:362–367
Mohammed NM, Mahfouz A, Achkar K, Rafie IM, Hajar R (2013) Contrast-induced Nephropathy. Heart Views 14:106–116
Kooiman J, Seth M, Share D, Dixon S, Gurm HS (2014) The association between contrast dose and renal complications post PCI across the continuum of procedural estimated risk. PLoS One 9:e90233
Jacobson B (1953) Dichromatic absorption radiography; dichromography. Acta Radiol 39:437–452
Hounsfield GN (1973) Computerized transverse axial scanning (tomography). 1. Description of system. Br J Radiol 46:1016–1022
McCollough CH, Leng S, Yu L, Fletcher JG (2015) Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology 276:637–653
van Ommen F, Bennink E, Vlassenbroek A et al (2018) Image quality of conventional images of dual-layer SPECTRAL CT: a phantom study. Med Phys 45:3031–3042
van Hamersvelt RW, Eijsvoogel NG, Mihl C et al (2018) Contrast agent concentration optimization in CTA using low tube voltage and dual-energy CT in multiple vendors: a phantom study. Int J Cardiovasc Imaging 34:1265–1275
Albrecht MH, Vogl TJ, Martin SS et al (2019) Review of clinical applications for virtual monoenergetic dual-energy CT. Radiology 293:260–271
Shuman WP, O’Malley RB, Busey JM, Ramos MM, Koprowicz KM (2017) Prospective comparison of dual-energy CT aortography using 70% reduced iodine dose versus single-energy CT aortography using standard iodine dose in the same patient. Abdom Radiol (NY) 42:759–765
Saltybaeva N, Jafari ME, Hupfer M, Kalender WA (2014) Estimates of effective dose for CT scans of the lower extremities. Radiology 273:153–159
Fei X, Du X, Yang Q et al (2008) 64-MDCT coronary angiography: phantom study of effects of vascular attenuation on detection of coronary stenosis. AJR Am J Roentgenol 191:43–49
Tan SK, Ng KH, Yeong CH et al (2019) Personalized administration of contrast medium with high delivery rate in low tube voltage coronary computed tomography angiography. Quant Imaging Med Surg 9:552–564
De Cecco CN, Schoepf UJ (2018) New contrast injection strategies for low kV and keV imaging. Appl Radiol 47:7–11
Horehledova B, Mihl C, Milanese G et al (2018) CT angiography in the lower extremity peripheral artery disease feasibility of an ultra-low volume contrast media protocol. Cardiovasc Intervent Radiol 41:1751–1764
Park JW, Kim SS, Lee JH, Park JH (2019) Feasibility of reduced radiation dose and iodine load in lower extremity computed tomography angiography. Hong Kong J Radiol 22:172–179
Qi L, Meinel FG, Zhou CS et al (2014) Image quality and radiation dose of lower extremity CT angiography using 70 kVp, high pitch acquisition and sinogram-affirmed iterative reconstruction. PLoS One 9:e99112
Ren H, Zhen Y, Gong Z, Wang C, Chang Z, Zheng J (2021) Feasibility of low-dose contrast media in run-off CT angiography on dual-layer spectral detector CT. Quant Imaging Med Surg 11:1796–1804
Almutairi A, Sun Z, Poovathumkadavi A, Assar T (2015) Dual energy CT angiography of peripheral arterial disease: feasibility of using lower contrast medium volume. PLoS One 10:e0139275
Davenport MS, Parikh KR, Mayo-Smith WW, Israel GM, Brown RK, Ellis JH (2017) Effect of fixed-volume and weight-based dosing regimens on the cost and volume of administered iodinated contrast material at abdominal CT. J Am Coll Radiol 14:359–370
Hendriks BM, Kok M, Mihl C, Bekkers SC, Wildberger JE, Das M (2016) Individually tailored contrast enhancement in CT pulmonary angiography. Br J Radiol 89:20150850
Ratnakanthan PJ, Kavnoudias H, Paul E, Clements WJ (2020) Weight-adjusted contrast administration in the computed tomography evaluation of pulmonary embolism. J Med Imaging Radiat Sci 51:451–461
Eijsvoogel NG, Hendriks BMF, Nelemans P et al (2020) Personalization of CM injection protocols in coronary computed tomographic angiography (People CT trial). Contrast Media Mol Imaging 2020:5407936
Acknowledgements
The authors thank our radiographer colleagues in the CT department for all their practical support, and for carrying out study examinations in the CT lab.
Funding
Open access funding provided by OsloMet - Oslo Metropolitan University
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Peter Mæhre Lauritzen.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• randomized controlled trial
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kristiansen, C.H., Thomas, O., Tran, T.T. et al. Halved contrast medium dose in lower limb dual-energy computed tomography angiography—a randomized controlled trial. Eur Radiol 33, 6033–6044 (2023). https://doi.org/10.1007/s00330-023-09575-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09575-3