Abstract
Objectives
Diabetes frequently results in cognitive impairment, but it is less clear if brain health is adversely affected during the prediabetic stage. Our aim is to identify possible changes in brain volume as measured by magnetic resonance imaging (MRI) in a large elderly population stratified according to level of “dysglycemia.”
Methods
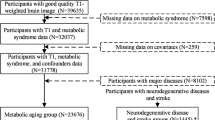
This is a cross-sectional study of 2144 participants (median age 69 years, 60.9% female) who underwent 3-T brain MRI. Participants were divided into 4 dysglycemia groups based on HbA1c levels (%): normal glucose metabolism (NGM) (< 5.7%), prediabetes (5.7 to < 6.5%), undiagnosed diabetes (6.5% or higher), and known diabetes (defined by self-report).
Results
Of the 2144 participants, 982 had NGM, 845 prediabetes, 61 undiagnosed diabetes, and 256 known diabetes. After adjustment for age, sex, education, body weight, cognitive status, smoking, drinking, and disease history, total gray matter volume was significantly lower among participants with prediabetes (0.41% lower, standardized β = − 0.0021 [95% CI − 0.0039, − 0.00039], p = 0.016), undiagnosed diabetes (1.4% lower, standardized β = − 0.0069 [95% CI − 0.012, − 0.002], p = 0.005), and known diabetes (1.1% lower, standardized β = − 0.0055 [95% CI − 0.0081, − 0.0029], p < 0.001) compared to the NGM group. After adjustment, total white matter volume and hippocampal volume did not differ significantly between the NGM group and either the prediabetes group or the diabetes group.
Conclusion
Sustained hyperglycemia may have deleterious effects on gray matter integrity even prior to the onset of clinical diabetes.
Key Points
• Sustained hyperglycemia has deleterious effects on gray matter integrity even prior to the onset of clinical diabetes.

Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- GMV:
-
Gray matter volume
- HV:
-
Hippocampal volume
- ICV:
-
Intracranial volume
- NGM:
-
Normal glucose metabolism
- WMV:
-
White matter volume
References
Bangen KJ, Beiser A, Delano-Wood L et al (2013) APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis 22:1361–1369
Cheng G, Huang C, Deng H, Wang H (2012) Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 42:484–491
Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM (1999) Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology 53:1937–1942
Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging S (2002) Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 51:1256–1262
Cooper C, Sommerlad A, Lyketsos CG, Livingston G (2015) Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry 172:323–334
Luchsinger JA (2010) Diabetes, related conditions, and dementia. J Neurol Sci 299:35–38
Moran C, Phan TG, Chen J et al (2013) Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 36:4036–4042
Schneider ALC, Selvin E, Sharrett AR et al (2017) Diabetes, prediabetes, and brain volumes and subclinical cerebrovascular disease on MRI: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Diabetes Care 40:1514–1521
Reitz C, Guzman VA, Narkhede A, DeCarli C, Brickman AM, Luchsinger JA (2017) Relation of dysglycemia to structural brain changes in a multiethnic elderly cohort. J Am Geriatr Soc 65:277–285
Dong S, Dongwei L, Zhang J, Liang J, Sun Z, Fang J (2019) Individuals in the prediabetes stage exhibit reduced hippocampal tail volume and executive dysfunction. Brain Behav 9:e01351
Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK (2016) Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomarker Insights 11:95–104
Association AD (2019) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care 42:S13–S28
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113
Ashburner J (2012) SPM: a history. Neuroimage 62:791–800
Ashburner J (2009) Computational anatomy with the SPM software. Magn Reson Imaging 27:1163–1174
Shattuck DW, Mirza M, Adisetiyo V et al (2008) Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 39:1064–1080
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’for medical statistics. Bone Marrow Transplant 48:452–458
Vogt NM, Hunt JF, Adluru N et al (2020) Cortical microstructural alterations in mild cognitive impairment and Alzheimer’s disease dementia. Cereb Cortex 30:2948–2960
Espeland MA, Bryan RN, Goveas JS et al (2013) Influence of type 2 diabetes on brain volumes and changes in brain volumes: results from the Women’s Health Initiative Magnetic Resonance Imaging studies. Diabetes Care 36:90–97
Falvey CM, Rosano C, Simonsick EM et al (2013) Macro-and microstructural magnetic resonance imaging indices associated with diabetes among community-dwelling older adults. Diabetes Care 36:677–682
Saczynski JS, Siggurdsson S, Jonsson PV et al (2009) Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care 32:1608–1613
Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5:64–74
Cholerton B, Baker LD, Craft S (2011) Insulin resistance and pathological brain ageing. Diabet Med 28:1463–1475
Wong TY, Cheung CM, Larsen M, Sharma S, Simo R (2016) Diabetic Retinopathy Nat Rev Dis Primers 2:16012
Sundermann EE, Thomas KR, Bangen KJ et al (2021) Prediabetes is associated with brain hypometabolism and cognitive decline in a sex-dependent manner: a longitudinal study of nondemented older adults. Front Neurol 12:144
Talbot K, Wang HY, Kazi H et al (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Investig 122:1316–1338
Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11:1006–1012
Van Loenhoud AC, Groot C, Vogel JW, Van Der Flier WM, Ossenkoppele R (2018) Is intracranial volume a suitable proxy for brain reserve? Alzheimer’s Res Ther 10:1–12
Jovicich J, Czanner S, Han X et al (2009) MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 46:177–192
Wonderlick JS, Ziegler DA, Hosseini-Varnamkhasti P et al (2009) Reliability of MRI-derived cortical and subcortical morphometric measures: effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage 44:1324–1333
Acknowledgements
The authors thank all the investigators and participants of this study.
Funding
This study has received funding by AMED (Japan Agency for Medical Research and Development) under Grant Number JP16dk0207025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shingo Kakeda.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• cross-sectional study/observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tatsuo, S., Watanabe, K., Ide, S. et al. Association of prediabetes with reduced brain volume in a general elderly Japanese population. Eur Radiol 33, 5378–5384 (2023). https://doi.org/10.1007/s00330-023-09509-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09509-z