Abstract
Objectives
To investigate the prognostic value of [18F]FDG PET/CT parameters in local recurrent nasopharyngeal carcinoma (lrNPC) and establish a prognostic tool for lrNPC patients based on these [18F]FDG PET/CT parameters.
Methods
A total of 358 lrNPC patients seen from 2010 to 2019 at Sun Yat-sen University Cancer Center with complete baseline characteristics and [18F]FDG PET/CT data were retrospectively analyzed. Maximal standardized uptake value (SUVmax), SUVmean, SUVpeak, metabolic tumor volume (MTV), total lesion glycolysis (TLG), and heterogeneity index (HI) for recurrent nasopharynx tumors were included. Cox regression analysis was performed to select candidate variables. Subsequently, a nomogram for predicting overall survival (OS) for lrNPC patients was developed and internally validated.
Results
Multivariate Cox analysis results suggested that age ≥ 47 years (hazard ratio (HR), 1.62 (1.18-2.24); p = 0.003),with smoking history (HR, 1.41 (1.01–1.98); p = 0.046), recurrent T stage {[rT3 vs rT1/2: HR, 1.81 (1.04–3.12); p = 0.037]; [rT4 vs rT1/2: HR, 2.46 (1.32–4.60); p = 0.005]}, and TLG {[37.1–184.3 vs ≤ 37.1: HR, 2.26 (1.49–3.42); p < 0.001]; [>184.3 vs ≤ 37.1: HR, 4.31 (2.50–7.43); p < 0.001]) were independent predictors of OS. A 4-factor nomogram was generated to stratify patients into 3 risk groups. This novel model showed good discrimination with a high C-index (0.752, 95%CI: 0.714–0.790). In addition, the calibration curves showed good agreement between the predicted probabilities and actual observations and decision curve analysis (DCA) suggested that the nomogram was useful for clinical decision-making.
Conclusions
Our study confirmed that [18F]FDG PET/CT parameters were valuable in predicting OS and PFS for lrNPC patients. The 4-factor prognostic model combing baseline patient characteristics with [18F]FDG PET/CT parameters for lrNPC patients had good discrimination, agreement, and clinical application potential.
Key Points
• [ 18 F]FDG PET/CT parameters were valuable in predicting OS and PFS for lrNPC patients.
• The novel 4-factor nomogram for lrNPC patients had good discrimination, agreement, and potential for clinical application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is a head and neck cancer with a distinct geographical distribution that is particularly prevalent in South China, Southeastern Asia, and North Africa [1]. Although intensity-modulated radiotherapy (IMRT) has attained excellent locoregional control rates, approximately 10% of NPC patients will experience local and/or regional lymph node recurrence after following primary treatment [2, 3]. Currently, local reirradiation or surgery achieves a good curative effect and significantly increases the overall survival (OS) of these patients [4,5,6,7,8]. However, for some lrNPC patients, the prognosis remains relatively poor, or serious side effects are experienced. Therefore, it is urgent in clinical practice to identify the different risk levels of patients for individualized treatment [2, 9].
Clinically, many guidelines recommend performing [18F]FDG PET/CT at the time of local recurrence because up to 20% of lrNPC patients have been reported to have concomitant distant metastasis [10,11,12,13]. In previous studies, some [18F]FDG PET/CT parameters, specifically, maximal standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were correlated with clinical outcomes for various cancers [14,15,16]. Previous models for prognostic stratification of recurrent NPC patients were established based on the gross tumor volume (GTV), prior radiotherapy-induced grade, synchronous nodal recurrence, the EBV-DNA level and baseline characteristics including age, physical state, hypertension, and the recurrent T-category [17,18,19,20]. However, it is unclear whether these [18F]FDG PET/CT parameters can predict the prognosis of patients with lrNPC. Therefore, our current study aimed to elucidate the role of these parameters in lrNPC and establish a survival model that combined these parameters with other important clinical prognosticators for tailoring lrNPC patients and tailoring individual therapy.
In our study, we aimed to investigate the prognostic value of [18F]FDG PET/CT parameters for predicting the OS and PFS of lrNPC patients, and the correlation between these parameters and clinically important factors of the recurrent TNM stage and the Epstein-Barr virus DNA (EBV-DNA) level. In addition, we intended to develop and evaluate an OS nomogram integrating PET/CT parameters and baseline characteristics for predicting the prognosis of lrNPC.
Materials and methods
Patient inclusion criteria
A total of 358 lrNPC patients were treated at Sun Yat-sen University Cancer Center (SYSUCC) from Nov 2010 to May 2019 with a complete pre-therapeutic baseline and 18F-FDG PET/CT data were retrospectively reviewed. Inclusion criteria were (1) lrNPC with or without regional lymph nodal metastasis, (2) no evidence of distant metastasis, (3) aged 18 to 70 years, and (4) diagnosis confirmed by either pathology or radiological findings and clinical symptoms. In addition, [18F]FDG PET/CT was performed less than 2 weeks before lrNPC treatment and a minimum of 24-month follow-up.
Data collection and ethics
Demographics and clinical information, such as age, sex, smoking history, drinking history, hypertension, NPC family history, ECOG performance score, prior treatments, recurrent TNM stage (the 8th Edition of the Union for International Cancer Control TNM staging system), and pre-treatment plasma EBV-DNA levels were collected. This study was approved by the Ethics Committee of Sun Yat-Sen University Cancer Center Health Authority (approved number: B2022-055-01) and was performed according to the ethical standards of the Declaration of Helsinki (as revised in 2013). Reporting of the study conforms to STROBE, along with references to STROBE and the broader EQUATOR guidelines. The study data underlying the findings of the current work were deposited at the Research Data Deposit platform (available at http://www.researchdata.org.cn/).
PET/CT image acquisition and analysis
[18F]FDG PET/CT scans were performed using a dedicated PET/CT system (Discovery ST16; GE Medical Systems, Milwaukee, WI, USA). Imaging was performed using a combination PET/CT scanner according to PET/CT tumor imaging guidelines [21]. Detailed information on PET/CT protocol was described in the previous study [22]. The reconstruction was performed using the ordered subset expectation maximization iterative algorithm (OSEM). [18F]FDG PET/CT parameters, including SUVmax, SUVpeak, SUVmean, and MTV were obtained from [18F]FDG PET images of the recurrent nasopharynx tumor using PMOD software (PMOD Technologies Ltd.). As in previous studies, an SUV of 2.5 was used as a threshold for lesion contouring [23, 24], and contours around the target lesion inside the boundaries were automatically generated. MTV was recorded at an SUV > 2.5 within the contouring margin, while benign lesions were excluded. Lastly, TLG was calculated as SUVmean × MTV [25], and HI was calculated as SUVmax/SUVmean [26].
Nomogram development, evaluation, and validation
Univariate Cox regression analyses were used to select candidate clinical predictors using a significant threshold of p < 0.1. Multivariable Cox analysis was performed to confirm the independent factors for the OS of lrNPC patients using the forward stepwise method. A nomogram was then developed based on this multivariate Cox proportional risk regression model. The discrimination of the model was evaluated using the Harrell Concordance Index (C-index). A Receiver operating characteristic (ROC) curve analysis was used to evaluate the sensitivity and specificity of this nomogram. Subsequently, the calibration plots of the nomogram were assessed by comparing the observed Kaplan-Meier estimates of survival probability to the nomogram-predicted survival probability. DCA curves were generated to assess the clinical application of the model. The predictive performance of the final model was internally validated using two-step bootstrap resampling procedures.
Statistical analysis
The primary outcome was OS, defined as the time from lrNPC diagnosis to all-cause death or censoring at the date of the last follow-up. Progression-free survival (PFS) was defined as the duration from the diagnosis date of lrNPC to the date of disease progression according to the RECIST 1.1 guidelines or all-cause death. The case deletion method was used to handle missing values in all explanatory variables. The best cut-off values of continuous variables were calculated by X-tile (Version3.6.1). Continuous variables are expressed as medians (IQRs), and categorical variables are expressed as numbers (percentages). The unpaired continuous variable data were compared using the Mann-Whitney test. These differential parameter levels at different clinical stages analysis used a one-way ANOVA. A linear correlation analysis was used to analyse the correlation between EBV-DNA levels and these 6 parameters. Associations between OS and potential prognostic factors were assessed by using the log-rank test in univariate analysis. All statistical analyses were undertaken by using R version-4.0.5, SPSS version-25.0, and GraphPad Prism version 9. All p < 0.05 (two-tailed) was considered statistically significant.
Results
Patient characteristics
Of the 358 patients included in this study. 258 were males, 100 were females, and the median age was 47 years (IQR: 40–55 years). The median values of SUVmax, SUVpeak, SUVmean, MTV, TLG, and HI were 10.1 g/mL (IQR: 6.6–14.3 g/mL), 6.8 g/mL (IQR: 4.7–10.4 g/mL), 4.2 g/mL (IQR: 3.6–5.1 g/mL), 9.5 mL (IQR:3.8–24.6 mL), 42.9 g/mL × mL (IQR: 14.8–117.0 g/mL × mL), and 2.3 (IQR: 1.8–2.7), respectively. The median follow-up time was 37.2 (IQR: 26.0–50.7) months. The median OS and PFS were 56.3 (IQR: 47.0–65.6) and 36.5 (IQR: 31.5–41.5) months, respectively. The 1-, 3-, and 5-year OS (PFS) rates were 90.2% (81.8%), 67.9% (50.5%), and 48.1% (31.5%), respectively. Table 1 demonstrated other patients’ characteristics, including smoking/drinking/NPC family history, ECOG performance score, comorbidity, EBV DNA levels, recurrent T/N/overall stage, and treatments. In addition, the causes of death are provided in Supplementary Results.
Correlation analysis
Correlations between [18F]FDG PET/CT parameters and clinical characteristics such as recurrent T stage, N stage, overall stage, and EBV-DNA levels (log10) were calculated. As shown in Figure S1, the more advanced the recurrent T stage and overall stage were, the higher the levels of these 6 PET/CT parameters (SUVmax, SUVpeak, SUVmean, TLG, MTV, and HI) were (all p < 0.0001). However, in the advanced recurrent N stage, only TLG and MTV increased with the advanced stage, possibly due to the number of patients with recurrent N3 (Figure S1G-L). Linear correlation analysis indicated that these 6 parameters were positively related to EBV-DNA level (R = 0.24–0.34, all p < 0.0001, Fig. 1A–F). In addition, patients with detectable EBV-DNA levels (EBV-DNA copy number > 0) had higher levels of these 6 parameters than patients with undetectable EBV-DNA levels (Fig. 1A–F, all p < 0.05).
Prognostic analysis
Univariate Cox regression analyses were performed to estimate the prognostic value of these PET/CT parameters in patients with lrNPC. As shown in Fig. 2 and Figure S2, these pretreatment PET/CT parameters included SUVmax, SUVpeak, SUVmean, MTV, TLG and HI were all associated with OS and PFS (all p < 0.05). The higher levels of these 6 parameters were, the worse the OS and PFS of lrNPC patients. In addition, some patients’ characteristics were also analyzed (Supplementary Results and Figure S3-4). Table 2 demonstrates bootstrap validation of the univariate Cox regression for PET/CT parameters and patient characteristics.
Kaplan–Meier survival curves comparing overall survival (OS) stratified by the cut-off SUVmax (a), SUVpeak (b), SUVmean (c), MTV (d), TLG (e), and HI (f). p values were calculated using the log-rank test. SUV: maximal standardized uptake value, MTV: metabolic tumor volume, TLG: total lesion glycolysis, HI: heterogeneity index
Subsequently, multivariate Cox regression was used to screen variables. As shown in Table 3, older (HR: 1.62, 95% CI: 1.18–2.24, p = 0.003), smoking history (HR: 1.41, 95% CI: 1.01–1.98, p = 0.046), advanced recurrent T stage (rT3 vs rT1/2: HR: 1.81, 95% CI: 1.04–3.12, p = 0.037; rT4 vs rT1/2: HR: 2.46, 95% CI: 1.32–4.60, p = 0.005) and a higher TLG value (37.1–184.3 vs ≤ 3.71: HR: 2.26, 95% CI: 1.49–3.42, p < 0.001; >184.3 vs ≤ 3.71: HR: 4.31, 95% CI: 2.50–7.43, p < 0.001) were identified as independent factors for worse OS of lrNPC patients.
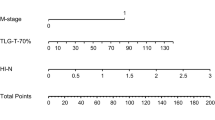
Model establishment and validation
These four indicators of age, smoking history, recurrent T stage, and TLG were selected for our model. Using these four regression coefficients, an OS nomogram for individualized 3-year OS estimation was developed (Fig. 3). Every predictor variable value was assigned a corresponding score according to a point scale. By adding up the score of each variable and locating the total score to the survival rate scale, OS probabilities could be estimated at the time points of 3 and 5 years. The C-index was 0.752 (95% CI: 0.714–0.790). The prognostic accuracy of the nomogram for 3- and 5-year OS was also assessed using ROC curves with areas under the curve (AUCs) of 0.793 and 0.791, respectively (Fig. 4A, B). The calibration curves of the nomogram for OS are shown in Fig. 4C, D which showed good agreement between the estimated outcomes and the observed outcomes. As shown in the DCA curves (Fig. 4E, F), using the nomogram to predict OS offered a net benefit over the “treat-none” or “treat-all” strategy, suggesting that the nomogram was useful in clinical decision-making.
Risk group stratification
The risk stratification in our cohort was based on the best cut-off values of the total score derived from the nomogram model. All patients were categorized into three risk groups: low-risk (total score < 98), intermediate-risk (total score 98–162), and high-risk (total score >162). The median OS times of the low-, intermediate-, and high-risk groups were 92.2 (IQR: 75.9–108.4), 34.5 (IQR: 25.5–43.4), and 17.7 (IQR: 12.0–23.4) months, respectively. The median PFS times of the low, intermediate, and high-risk groups were 53.7 (IQR: 42.5–64.9), 26.5 (IQR: 21.4–31.7), and 12.5 (IQR: 7.5–17.5) months, respectively. In addition, the 3-year (5-year) OS rates were 87.5% (69.3%), 49.4% (26.3%), and 27.3% (9.9%) among these 3 risk groups, respectively. The 3-year (5-year) PFS rates were 66.3% (46.7%), 36.8% (18.7%), and 16.3% (0) in these 3 risk groups, respectively. Figure 5A shows the Kaplan–Meier survival curves for OS of different risk groups. The intermediate-risk (HR1: 3.56, 95%CI: 2.49–5.08, p < 0.001) and high-risk (HR2: 8.19, 95%CI: 5.30–12.65, p < 0.001) groups had a worse prognosis than the low-risk group, and the high-risk group also had a worse survival than the intermediate-risk group (HR3: 2.15, 95%CI: 1.44–3.22, p < 0.001). Similar results in PFS can be seen in Fig. 5B. Significant differences were also observed between the OS and PFS of patients with different risk levels, which further confirmed that our nomogram could appropriately stratify different risk lrNPC patients and may tailor individual therapy.
Discussion
lrNPC presents a challenging treatment situation and the prognosis of patients is difficult to predict [5,6,7]. Therefore, developing a tool to stratify the prognosis of patients with lrNPC is of great clinical importance. In the clinic, it is routine for lrNPC patients to receive a [18F]FDG PET/CT since approximately 20% of patients have concomitant distant metastasis at the time of local recurrence [27, 28]. Previous studies have reported that PET/CT parameters such as SUVmax, MTV, and TLG may predict cancer prognosis [28,29,30,31,32]. Therefore, it is valuable to understand the correlation between PET/CT parameters and the prognosis of lrNPC. In this study, we found that higher PET/CT parameters, including SUVmax, SUVpeak, SUVmean, MTV, TLG, and HI, were significantly associated with advanced recurrent T stage, higher EBV-DNA levels, and worse OS and PFS of lrNPC patients. Based on these findings, a four-factor OS nomogram integrating age, smoking history, recurrent T stage, and TLG was developed and evaluated for predicting the prognosis of patients with lrNPC. This nomogram had good discrimination, calibration, and clinical application value. We also found that the nomogram could discriminate among high-risk, intermediate-risk, and low-risk patients with lrNPC. High-risk lrNPC patients may need more aggressive treatment, such as immunotherapy, because of the worst survival among different risk lrNPC patients.
[18F]FDG PET/CT, as the most widely used, functional imaging technique, can provide metabolic information for the entire tumor [29]. Furthermore, [18F]FDG FDG-PET has been shown to be superior to MRI in detecting residual/recurrent NPC with higher sensitivity, specificity, and accuracy [30]. The [18F]FDG standardized uptake value (SUV) has been reported to be associated with both the density of tumor cells and the glucose metabolic rate [31]. Lee SW et al found that NPC, which had a higher [18F]FDG uptake, had a significantly lower 3-year disease-free survival [32]. In addition, MTV and TLG derived from [18F]FDG PET/CT are strong predictors of OS in patients treated with radiotherapy, chemotherapy, and immunotherapy in several types of cancers [33,34,35,36,37]. Chan et al found that SUVmax can predict the surgical outcome of nasopharyngectomy and cervical lymphadenectomy for recurrent NPC [38]. Consistent with prior research, parameters including SUVmax, SUVpeak, SUVmean, MTV, TLG, and HI were found to be associated with the prognosis of lrNPC in our study (Fig. 1). Interestingly, these 6 parameters were also correlated with the recurrent T stage, as well as the serum EBV-DNA level, which was previously reported to be closely associated with the prognosis of NPC patients. TLG is a composite parameter representing tumor volume and metabolic status [39, 40]. In the multivariate Cox regression analysis of the current study, TLG was the only independent prognostic factor of [18F]FDG PCT/CT parameters for lrNPC patients.
In the era of intensity-modulated radiotherapy, approximately 10% of NPC patients still develop local or regional recurrence [3, 41]. Currently, the American Joint Committee Cancer (AJCC) recurrent TNM staging system (rTNM) is widely used to predict clinical outcomes for these patients. However, the usefulness of rTNM staging is limited, since these outcomes vary among patients within the same stage [6, 38, 42, 43]. Therefore, precise risk stratification to guide individualized treatment for these patients is an urgent clinical problem to be solved. Li et al constructed comprehensive prognostic models using age, GTV, prior RT-induced grade ≥ 3 toxicities, rT stage, and repeat IMRT equivalent dose in 2-Gy fractions (EQD2) to personalize recommendations for salvage intensity-modulated radiotherapy (IMRT) in patients with local recurrent NPC [19]. Similarly, Tian et al developed a prognostic-score model for classified local recurrent NPC patients suitable for undergoing reirradiation with IMRT. Model variables included Karnofsky performance status (KPS), age, late complications, recurrent T stage, synchronous nodal recurrence, and GTV [17]. Sun et al established a prognostic model integrating demographic and EBV-DNA levels [20]. However, this simple model did not have very good discrimination, with a C-index was 0.687, compared to ours of 0.752. Additionally, EBV-DNA measurement is difficult for many basic-level hospitals to achieve. In the current study, PET/CT parameters were strongly correlated with the prognosis of lrNPC (Fig. 2). However, no prognostic model based on these PET/CT parameters has been reported in the literature. Therefore, a prognostic model based on these parameters is valuable. Finally, we develop a 4-factor OS nomogram for lrNPC patients combining TLG and 3 patient characteristics including age, smoking history, and recurrent T stage. In our nomogram, smoking was one of the poor prognosis factors of lrNPC patients, which was consistent with previous reports [44, 45]. However, the poor results of the internal bootstrap validation may be due to the influence of sex on the sampling process.
Our study has several limitations. First, it was retrospective in design and therefore was prone to selection bias. Second, although our model exhibited good discrimination and agreement, the model lacked external validation. In addition, our model requires further validation in prospective studies and multicenter clinical trials.
The [18F]FDG PET/CT parameters were valuable in predicting OS and PFS in lrNPC patients, and our four-factor nomogram integrating clinical characteristics and [18F]FDG PET/CT parameters to predict OS for lrNPC patients had good discrimination, agreement, and potential for clinical application.
Abbreviations
- [18F]FDG:
-
18F-Fluoro-2-deoxy-2-D-glucose
- AUC:
-
Area under the curve
- CI:
-
Confidence intervals
- C-index:
-
Harrell concordance index
- DCA:
-
Decision curve analysis
- EBV:
-
Epstein-Barr virus
- ECOG:
-
The Eastern Cooperative Oncology Group
- EQD2:
-
Equivalent dose in 2-Gy fractions
- GTV:
-
Gross tumor volume
- HI:
-
Heterogeneity index
- HR:
-
Hazard ratio
- IMRT:
-
Intensity-modulated radiotherapy
- KPS:
-
Karnofsky performance status
- LrNPC:
-
Local recurrent nasopharyngeal carcinoma
- MTV:
-
Metabolic tumor volume
- OS:
-
Overall survival
- PET/CT:
-
positron emission tomography/computed tomography
- PFS:
-
Progression-free survival
- RECIST:
-
The response evaluation criteria in solid tumors
- ROC:
-
Receiver operating characteristic
- SUVmax:
-
Maximal standardized uptake value
- SUVmean:
-
Mean standardized uptake value
- SUVpeak:
-
Peak standardized uptake value
- TLG:
-
Total lesion glycolysis
References
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J (2019) Nasopharyngeal carcinoma. Lancet 394:64–80
Lee AWM, Ng WT, Chan JYW et al (2019) Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev 79:101890
Setton J, Han J, Kannarunimit D et al (2016) Long-term patterns of relapse and survival following definitive intensity-modulated radiotherapy for non-endemic nasopharyngeal carcinoma. Oral Oncol 53:67–73
Ng WT, Soong YL, Ahn YC et al (2021) International recommendations on reirradiation by intensity modulated radiation therapy for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 110:682–695
Leong YH, Soon YY, Lee KM, Wong LC, Tham IWK, Ho FCH (2018) Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: a meta-analysis. Head Neck 40:622–631
Hua YJ, Han F, Lu LX et al (2012) Long-term treatment outcome of recurrent nasopharyngeal carcinoma treated with salvage intensity modulated radiotherapy. Eur J Cancer 48:3422–3428
Yang J, Song X, Sun X et al (2020) Outcomes of recurrent nasopharyngeal carcinoma patients treated with endoscopic nasopharyngectomy: a meta-analysis. Int Forum Allergy Rhinol 10:1001–1011
You R, Zou X, Hua YJ et al (2015) Salvage endoscopic nasopharyngectomy is superior to intensity-modulated radiation therapy for local recurrence of selected T1-T3 nasopharyngeal carcinoma – a case-matched comparison. Radiother Oncol 115:399–406
Perri F, Della Vittoria Scarpati G, Caponigro F et al (2019) Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther 12:1583–1591
Bossi P, Chan AT, Licitra L et al (2021) Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up(†). Ann Oncol 32:452–465
Colevas AD, Yom SS, Pfister DG et al (2018) NCCN guidelines insights: head and neck cancers, Version 1.2018. J Natl Compr Canc Netw 16:479–490
Tang LL, Chen YP, Chen CB et al (2021) The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond) 41:1195–1227
Wei J, Pei S, Zhu X (2016) Comparison of [18F]FDG PET/CT, MRI and SPECT in the diagnosis of local residual/recurrent nasopharyngeal carcinoma: a meta-analysis. Oral Oncol 52:11–17
Salazar A, Júnior EP, Salles PGO, Silva-Filho R, Reis EA, Mamede M (2019) (18)F-FDG PET/CT as a prognostic factor in penile cancer. Eur J Nucl Med Mol Imaging 46:855–863
Vallius T, Hynninen J, Kemppainen J et al (2018) (18)F-FDG-PET/CT based total metabolic tumor volume change during neoadjuvant chemotherapy predicts outcome in advanced epithelial ovarian cancer. Eur J Nucl Med Mol Imaging 45:1224–1232
Chen YH, Chang KP, Chu SC et al (2019) Value of early evaluation of treatment response using (18)F-FDG PET/CT parameters and the Epstein-Barr virus DNA load for prediction of outcome in patients with primary nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging 46:650–660
Tian YM, Tian YH, Zeng L et al (2014) Prognostic model for survival of local recurrent nasopharyngeal carcinoma with intensity-modulated radiotherapy. Br J Cancer 110:297–303
Yue Q, Zhang M, Chen Y, Zheng D, Chen Y, Feng M (2018) Establishment of prognostic factors in recurrent nasopharyngeal carcinoma patients who received salvage intensity-modulated radiotherapy: a meta-analysis. Oral Oncol 81:81–88
Li YQ, Tian YM, Tan SH et al (2018) Prognostic model for stratification of radioresistant nasopharynx carcinoma to curative salvage radiotherapy. J Clin Oncol 36:891–899
Sun XS, Liang YJ, Jia GD et al (2020) Establishment of a prognostic nomogram to identify optimal candidates for local treatment among patients with local recurrent nasopharyngeal carcinoma. Oral Oncol 106:104711
Delbeke D, Coleman RE, Guiberteau MJ et al (2006) Procedure guideline for tumor imaging with [18F]FDG PET/CT 1.0. J Nucl Med 47:885–895
Peng H, Dong D, Fang MJ et al (2019) Prognostic value of deep learning PET/CT-based radiomics: potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin Cancer Res 25:4271–4279
Lin HC, Chan SC, Cheng NM et al (2020) Pretreatment (18)F-FDG PET/CT texture parameters provide complementary information to Epstein-Barr virus DNA titers in patients with metastatic nasopharyngeal carcinoma. Oral Oncol 104:104628
Chung MK, Jeong HS, Park SG et al (2009) Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 15:5861–5868
Larson SM, Erdi Y, Akhurst T et al (1999) Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The Visual Response Score and the Change in Total Lesion Glycolysis. Clin Positron Imaging 2:159–171
Salamon J, Derlin T, Bannas P et al (2013) Evaluation of intratumoural heterogeneity on [18F]FDG PET/CT for characterization of peripheral nerve sheath tumors in neurofibromatosis type 1. Eur J Nucl Med Mol Imaging 40:685–692
Chan OS, Sze HC, Lee MC et al (2017) Reirradiation with intensity-modulated radiotherapy for locally recurrent T3 to T4 nasopharyngeal carcinoma. Head Neck 39:533–540
Lee AW, Law SC, Foo W et al (1993) Retrospective analysis of patients with nasopharyngeal carcinoma treated during 1976-1985: survival after local recurrence. Int J Radiat Oncol Biol Phys 26:773–782
Manca G, Vanzi E, Rubello D et al (2016) (18)F-FDG PET/CT quantification in head and neck squamous cell cancer: principles, technical issues and clinical applications. Eur J Nucl Med Mol Imaging 43:1360–1375
Yen RF, Hung RL, Pan MH et al (2003) 18-fluoro-2-deoxyglucose positron emission tomography in detecting residual/recurrent nasopharyngeal carcinomas and comparison with magnetic resonance imaging. Cancer 98:283–287
Huang SC (2000) Anatomy of SUV. Standardized uptake value. Nucl Med Biol 27:643–646
Lee SW, Nam SY, Im KC et al (2008) Prediction of prognosis using standardized uptake value of 2-[(18)F] fluoro-2-deoxy-d-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother Oncol 87:211–216
Ren S, Zhu X, Zhang A, Li D, Zuo C, Zhang H (2020) Prognostic value of [18F]FDG PET /CT metabolic parameters in patients with locally advanced pancreatic cancer treated with stereotactic body radiation therapy. Cancer Imaging 20:22
Son SH, Kang SM, Jeong SY et al (2016) Prognostic value of volumetric parameters measured by pretreatment 18F FDG PET/CT in patients with cutaneous malignant melanoma. Clin Nucl Med 41:e266–e273
Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E (2019) FDG PET/CT for assessing tumor response to immunotherapy : report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging 46:238–250
Anwar H, Sachpekidis C, Winkler J et al (2018) Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging 45:376–383
Humbert O, Cadour N, Paquet M et al (2020) (18)FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging 47:1158–1167
Chan JY, Chow VL, Mok VW, Ho AC, Wei WI (2012) Prediction of surgical outcome using plasma Epstein-Barr virus dna and (18)F-FDG PET-CT scan in recurrent nasopharyngeal carcinoma. Head Neck 34:541–545
Pak K, Cheon GJ, Nam HY et al (2014) Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med 55:884–890
Reynolds JC, Maass-Moreno R, Thomas A et al (2020) (18)F-FDG PET Assessment of malignant pleural mesothelioma: total lesion volume and total lesion glycolysis-the central role of volume. J Nucl Med 61:1570–1575
Lee AW, Ng WT, Chan LL et al (2014) Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol 110:377–384
You R, Zou X, Wang SL et al (2015) New surgical staging system for patients with recurrent nasopharyngeal carcinoma based on the AJCC/UICC rTNM classification system. Eur J Cancer 51:1771–1779
Tian YM, Guan Y, Xiao WW et al (2016) Long-term survival and late complications in intensity-modulated radiotherapy of locally recurrent T1 to T2 nasopharyngeal carcinoma. Head Neck 38:225–231
Lin JH, Jiang CQ, Ho SY et al (2015) Smoking and nasopharyngeal carcinoma mortality: a cohort study of 101,823 adults in Guangzhou, China. BMC Cancer 15:906
Lin JH, Wen CP, Jiang CQ et al (2021) Smoking and nasopharyngeal cancer: individual data meta-analysis of six prospective studies on 334 935 men. Int J Epidemiol 50:975–986
Funding
This study was funded by grants from the National Natural Science Foundation of China (No. 81425018, No. 81672868), the Sci-Tech Project Foundation of Guangzhou City (201707020039), the Sun Yat-sen University Clinical Research 5010 Program (No. 2019023), the Special Support Plan of Guangdong Province (No. 2014TX01R145), the Natural Science Foundation of Guangdong Province for Distinguished Young Scholar (No. 2018B030306001), and the Pearl River S&T Nova Program of Guangzhou (No. 201806010135).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Chen Qiuyan.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Statistics and biometry
One of the authors has significant statistical expertise (Dr. Ning Kang).
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
This study was approved by the Ethics Committee of Sun Yat-Sen University Cancer Centre Health Authority (approved number: B2022-055-01).
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1614 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dongxiang, W., Liting, L., Yujing, L. et al. Prediction of outcomes in patients with local recurrent nasopharyngeal carcinoma: development and validation of a four-factor prognostic model integrating baseline characteristics and [18F]FDG PET/CT parameters. Eur Radiol 33, 2840–2849 (2023). https://doi.org/10.1007/s00330-022-09232-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09232-1