Abstract
Objectives
The objective of this study was to use computed tomography (CT) imaging to quantify chemotherapy-induced changes in body composition (BC) in pediatric, adolescent, and young adult (AYA) patients with lymphoma and to compare image-derived changes in BC measures to changes in traditional body mass index (BMI) measures.
Methods
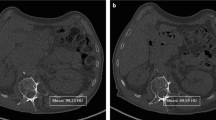
Skeletal muscle (SkM), subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT) volumes were manually segmented using low-dose CT images acquired from a 10-year retrospective, single-site cohort of 110 patients with lymphoma. CT images and BMI percentiles (BMI%) were acquired from baseline and first therapeutic follow-up. CT image segmentation was performed at vertebral level L3 using 5 consecutive axial CT images.
Results
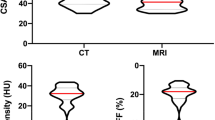
CT imaging detected significant treatment-induced changes in BC measures from baseline to first follow-up time points, with SAT and VAT significantly increasing and SkM significantly decreasing. BMI% measures did not change from baseline to first follow-up and were not significantly correlated with changes in image-derived BC measures. Patients who were male, younger than 12 years old, diagnosed with non-Hodgkin’s lymphoma, and presented with stage 3 or 4 disease gained more adipose tissue and lost more SkM in response to the first cycle of treatment compared to their clinical counterparts.
Conclusions
Standard of care CT imaging can quantify treatment-induced changes in BC that are not reflected by traditional BMI assessment. Image-based monitoring of BC parameters may offer personalized approaches to lymphoma treatment for pediatric and AYA patients by guiding cancer treatment recommendations and subsequently enhance clinical outcomes.
Key Points
• Standard of care low-dose CT imaging quantifies chemotherapy-induced changes in body composition in pediatric, adolescent, and young adults with lymphoma.
• Body mass index could not detect changes in body composition during treatment that were quantified by CT imaging.
• Pediatric and AYA patients who were male, younger than 12 years old, and diagnosed with non-Hodgkin’s lymphoma, and presented with stage 3 or 4 disease gained more adipose tissue and lost more skeletal muscle tissue in response to the first cycle of treatment compared to their clinical counterparts.




Similar content being viewed by others
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- AYA:
-
Adolescent and young adult
- BC:
-
Body composition
- BMI:
-
Body mass index
- CT :
-
Computed tomography
- DXA:
-
Dual-energy X-ray absorptiometry
- MR:
-
Magnetic resonance
- SAT:
-
Subcutaneous adipose tissue
- SkM:
-
Skeletal muscle
- VAT :
-
Visceral adipose tissue
References
Caan BJ, Cespedes Feliciano EM, Prado CM et al (2018) Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 4:798–804
Ryan AM, Prado CM, Sullivan ES, Power DG, Daly LE (2019) Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition 67-68:110539
Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM (2018) Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle 9:315–325
Cespedes Feliciano EM, Chen WY, Lee V et al (2020) Body composition, adherence to anthracycline and taxane-based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol 6:264–270
Thompson PA, Rosner GL, Matthay KK et al (2009) Impact of body composition on pharmacokinetics of doxorubicin in children: a Glaser Pediatric Research Network study. Cancer Chemother Pharmacol 64:243–251
Prado CM, Maia YL, Ormsbee M, Sawyer MB, Baracos VE (2013) Assessment of nutritional status in cancer--the relationship between body composition and pharmacokinetics. Anticancer Agents Med Chem 13:1197–1203
Prado CM, Lima IS, Baracos VE et al (2011) An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol 67:93–101
Prado CM, Baracos VE, McCargar LJ et al (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13:3264–3268
Joffe L, Schadler KL, Shen W, Ladas EJ (2019) Body composition in pediatric solid tumors: state of the science and future directions. J Natl Cancer Inst Monogr 2019:144–148
Kaestner SA, Sewell GJ (2007) Chemotherapy dosing part I: scientific basis for current practice and use of body surface area. Clin Oncol (R Coll Radiol) 19:23–37
Orgel E, Mueske NM, Sposto R, Gilsanz V, Freyer DR, Mittelman SD (2018) Limitations of body mass index to assess body composition due to sarcopenic obesity during leukemia therapy. Leuk Lymphoma 59:138–145
Murphy AJ, White M, Davies PS (2010) Body composition of children with cancer. Am J Clin Nutr 92:55–60
Collins L, Beaumont L, Cranston A, Savoie S, Nayiager T, Barr R (2017) Anthropometry in long-term survivors of acute lymphoblastic leukemia in childhood and adolescence. J Adolesc Young Adult Oncol 6:294–298
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Prado CM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Mei KL, Batsis JA, Mills JB, Holubar SD (2016) Sarcopenia and sarcopenic obesity: do they predict inferior oncologic outcomes after gastrointestinal cancer surgery? Perioper Med (Lond) 5:30
Yip C, Dinkel C, Mahajan A, Siddique M, Cook GJ, Goh V (2015) Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 6:489–497
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Murphy AJ, White M, Elliott SA, Lockwood L, Hallahan A, Davies PS (2015) Body composition of children with cancer during treatment and in survivorship. Am J Clin Nutr 102:891–896
den Hoed MA, Pluijm SM, de Groot-Kruseman HA et al (2015) The negative impact of being underweight and weight loss on survival of children with acute lymphoblastic leukemia. Haematologica 100:62–69
Brinksma A, Sanderman R, Roodbol PF et al (2015) Malnutrition is associated with worse health-related quality of life in children with cancer. Support Care Cancer 23:3043–3052
Santoro A, Guidarelli G, Ostan R et al (2019) Gender-specific association of body composition with inflammatory and adipose-related markers in healthy elderly Europeans from the NU-AGE study. Eur Radiol 29:4968–4979
Lee SY, Gallagher D (2008) Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care 11:566–572
Williams JE, Wells JC, Wilson CM, Haroun D, Lucas A, Fewtrell MS (2006) Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J Clin Nutr 83:1047–1054
Salinas-Miranda E, Deniffel D, Dong X et al (2021) Prognostic value of early changes in CT-measured body composition in patients receiving chemotherapy for unresectable pancreatic cancer. Eur Radiol 31:8662–8670
Peng YC, Wu CH, Tien YW, Lu TP, Wang YH, Chen BB (2021) Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur Radiol 31:2472–2481
Joffe L, Dwyer S, Glade Bender JL, Frazier AL, Ladas EJ (2019) Nutritional status and clinical outcomes in pediatric patients with solid tumors : a systematic review of the literature. Semin Oncol 46:48–56
Shen W, Punyanitya M, Wang Z et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 97:2333–2338
Yang HR, Choi HS (2019) A prospective study on changes in body composition and fat percentage during the first year of cancer treatment in children. Nutr Res Pract 13:214–221
Suzuki D, Kobayashi R, Sano H, Hori D, Kobayashi K (2018) Sarcopenia after induction therapy in childhood acute lymphoblastic leukemia: its clinical significance. Int J Hematol 107:486–489
Murphy AJ, Wells JC, Williams JE, Fewtrell MS, Davies PS, Webb DK (2006) Body composition in children in remission from acute lymphoblastic leukemia. Am J Clin Nutr 83:70–74
Rayar M, Webber CE, Nayiager T, Sala A, Barr RD (2013) Sarcopenia in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 35:98–102
Mueske NM, Mittelman SD, Wren TAL, Gilsanz V, Orgel E (2019) Myosteatosis in adolescents and young adults treated for acute lymphoblastic leukemia. Leuk Lymphoma 60:3146–3153
Armenian SH, Iukuridze A, Teh JB et al (2020) Abnormal body composition is a predictor of adverse outcomes after autologous haematopoietic cell transplantation. J Cachexia Sarcopenia Muscle 11:962–972
Marriott CJC, Beaumont LF, Farncombe TH et al (2018) Body composition in long-term survivors of acute lymphoblastic leukemia diagnosed in childhood and adolescence: a focus on sarcopenic obesity. Cancer 124:1225–1231
Ooi PH, Thompson-Hodgetts S, Pritchard-Wiart L, Gilmour SM, Mager DR (2020) Pediatric sarcopenia: a paradigm in the overall definition of malnutrition in children? JPEN J Parenter Enteral Nutr 44:407–418
Liu J, Yan Y, Xi B et al (2019) Skeletal muscle reference for Chinese children and adolescents. J Cachexia Sarcopenia Muscle 10:155–164
Joffe L, Shen W, Shadid G, Jin Z, Ladas EJ (2021) Skeletal muscle and adipose tissue changes in the first phase of treatment of pediatric solid tumors. Cancer Med 10:15–22
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006
Papademetris X, Jackowski MP, Rajeevan N et al (2006) BioImage Suite: An integrated medical image analysis suite: an update. Insight J 2006:209
van Vugt JL, Levolger S, Gharbharan A et al (2017) A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle 8:285–297
Centers for Disease Control and Prevention. Healthy weight, about child and teen BMI. Available via https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html. Accessed 09/13/2021
Lee SJ, Liu J, Yao J, Kanarek A, Summers RM, Pickhardt PJ (2018) Fully automated segmentation and quantification of visceral and subcutaneous fat at abdominal CT: application to a longitudinal adult screening cohort. Br J Radiol 91:20170968
Xiao DY, Luo S, O'Brian K et al (2016) Longitudinal body composition changes in diffuse large B-cell lymphoma survivors: a retrospective cohort study of United States Veterans. J Natl Cancer Inst 108:djw145
Acknowledgements
We would like to acknowledge Dariya Hardisky for her help with collecting the patient images.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Mitchel Stacy.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tram, N.K., Chou, TH., Ettefagh, L.N. et al. Quantification of chemotherapy-induced changes in body composition in pediatric, adolescent, and young adult lymphoma using standard of care CT imaging. Eur Radiol 32, 7270–7277 (2022). https://doi.org/10.1007/s00330-022-09048-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09048-z