Abstract
Objectives
Intravenous application of contrast media is part of a wide spectrum of diagnostic procedures for better imaging quality. Clinical avoidance of contrast-enhanced imaging is an ever-present quandary in patients with impaired kidney function. The objective of this study was to estimate the risk for acute kidney injury (AKI), dialysis and mortality among patients undergoing contrast-enhanced CT compared to propensity score–matched controls (i.e. contrast-unenhanced CT). Selected cohort studies featured high-risk patients with advanced kidney disease and critical illness.
Methods
This review was designed to conform to the Preferred Reporting Items in Systematic Reviews and Meta-Analysis (PRISMA) guidelines. PubMed was searched from August 2021 to November 2021 for all-language articles without date restriction. A random-effects model (DerSimonian and Laird method) was used for meta-analysis.
Results
Twenty-one articles were included, comprising data of 169,455 patients. The overall risk of AKI was similar in the contrast-enhanced and unenhanced groups (OR: 0.97 [95% CI: 0.85; 1.11], p = 0.64), regardless of baseline renal function and underlying disease. Substantial heterogeneity was detected (I2 = 90%, p ≤ 0.0001). Multivariable logistic regression identified hypertension (p = 0.03) and estimated glomerular filtration rate (eGFR) ≤ 30 mL/min/1.73 m2 (p = 0.0001) as factors associated with greater risk of post-contrast AKI.
Conclusions
Based on propensity score–matched pairs obtained from 21 cohort studies, we found no evidence for increased risk for AKI, dialysis or mortality after contrast-enhanced CT among patients with eGFR ≥ 45 mL/min/1.73 m2. In congruence with the emerging evidence in the literature, caution should be exercised in patients with hypertension and eGFR ≤ 30 mL/min/1.73 m2.
Key Points
• The application of contrast media for medical imaging is not associated with higher odds for AKI, induction of renal replacement therapy, or mortality. Many comorbidities traditionally associated with greater risk for acute kidney injury do not appear to predispose for renal decline after contrast media exposure.
• Underlying hypertension and eGFR less than or equal to 30 mL/min/1.73 m2 seem to predispose for post-contrast acute kidney injury.
• Propensity score matching cannot account for unmeasured influences on AKI incidence, which needs to be addressed in the interpretation of results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contrast-induced nephropathy (CIN), also known as contrast-associated acute kidney injury (CA-AKI) or contrast-induced acute kidney injury (CI-AKI), is defined as a rapid decline of renal function within days following intravascular exposure to contrast media (CM) [1, 2]. CA-AKI is traditionally suggested to be a leading cause of hospital-acquired acute kidney injury (AKI), presenting 12% of all cases [3]. The absolute and relative definitions of CA-AKI are diverse. Most commonly, it is characterized by an absolute increase in serum creatinine (SCr) levels of ≥ 0.3 mg/dL or ≥ 0.5 mg/dL from baseline. It can also be defined as a relative SCr increase of more than 25% of baseline or 1.5 times baseline within 1–3 or 4–5 days after intravenous or intra-arterial CM application [4,5,6]. By definition, no factors other than previous CM exposure can provide sufficient explanation for the renal decline [7, 8]. Post-contrast AKI is strongly associated with short- and long-standing adverse and potentially irreversible outcomes [9, 10].
Contrast-enhanced computed tomography (CT) is an indispensable component of medical imaging. Although low- and iso-osmolar contrast agents are generally considered safe, their intravenous administration for greater imaging quality and diagnostic accuracy has been assumed one of the most frequent causes of AKI in clinical practice [7].
However, numerous cohort studies have challenged this historic belief by using the propensity score (PS) to match CM–exposed subjects with unexposed controls. Propensity score matching (PSM) is an analytical approach to estimate the weight of CM exposure on the incidence of AKI [11]. Applying logistic regression, patients are matched according to similar distributions of baseline characteristics [11, 12]. Studies applying the PS have revealed equal rates of AKI in matched cohorts (i.e. CM exposed and unexposed), pointing out the underestimated role of underlying comorbidities in the development of AKI. Thus, it is suggested that the dreaded deterioration of kidney function following contrast-enhanced imaging might have been falsely attributed to CM rather than the susceptibility of particular patient collectives.
The objective of this systematic review and meta-analysis was to determine the risks of acute nephropathy in patients undergoing contrast-enhanced CT compared with demographically similar controls undergoing contrast-unenhanced CT. As secondary outcomes of interest, we evaluated the risks of dialysis and mortality in patients with CM–enhanced and unenhanced CT.
Materials and methods
Protocol and registration
The protocol for this systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) under the identification number 197088, and was accepted on September 6, 2020.
Data sources and search
Two investigators (M.O. and B.M.W.S.) reviewed all English-language publications in Cochrane Library, PubMed and MEDLINE using the search terms “propensity score AND contrast media“ and “(AKI OR nephropathy) AND (iodinated contrast OR contrast media OR CT OR angiography) AND propensity score” with no date restrictions. Data extraction was performed until November 2021, using a predefined set of inclusion and exclusion criteria. In case of differing results between both researchers, a third (K.W.) and a fourth (E.D.) investigator reviewed and adjudicated the results.
Eligibility criteria
All studies reporting on the effects of CM exposure on AKI incidence using a propensity score–matching model were considered for inclusion. Eligible studies required two arms: one group of patients undergoing CM–enhanced CT and a control group undergoing unenhanced CT. Studies were included for meta-analysis if AKI was defined by Acute Kidney Injury Network (AKIN); Kidney Disease: Improving Global Outcomes (KDIGO); Risk, Injury, Failure, Loss of kidney function, and End-stage Kidney Disease (RIFLE); or contrast-induced nephropathy (CIN) criteria [13,14,15,16]. The presence of SCr measurements or glomerular filtration rates (GFRs) before and after CT scans was required for inclusion. No age restriction was applied. Trials comparing different doses of the same intervention or applying re-randomization of the same sample (i.e. crossover design) and trials that lacked either PSM or a proper comparator arm were excluded. Studies reporting on solitary kidneys or procedures other than CT scans were also excluded. The same applied to patient cohorts undergoing multiple CT scans within 72 h. Systematic reviews and meta-analyses were excluded; however, the references of all identified reviews were searched for additional citations [17,18,19].
Study selection and data collection process
The data of selected studies was independently extracted by three investigators (M.O., M.M.G., C.V.B.). In case of discrepancies on study findings, outcomes were discussed (with E.D., supervised by B.M.W.S. and K.W.), and consensus was established. Points of discussion included the handling of articles with mixed CM pathways (i.e. intravenous and intra-arterial), overlapping study populations, differing matching models and the consideration of multiple AKI criteria in the same study.
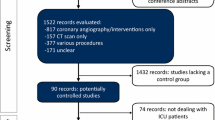
Our initial search identified 297 publications (Fig. 1). After duplicates were removed, the remaining 99 articles were reviewed by title, leading to the exclusion of 58 records. The remaining 41 articles were screened by abstract. All papers fulfilling the inclusion criteria (n = 26) were assessed by full-text review. Potential doubling or reutilization of study populations was thoroughly checked. Ultimately, 21 articles were selected for final data extraction, comprising data of 169,455 patients undergoing CT.
Data items and statistical analyses
The number of subjects and the study type were retrieved for each study (Table 1). Additionally, the applied matching ratios (i.e. 1:1, 1:3), type of study population (e.g. general population, septic patients) and applied AKI definition (i.e. AKIN, KDIGO, RIFLE or CIN) were identified. The Newcastle–Ottawa Scale was implemented to assess the risk of bias [20]. The calculated scores were converted to the Agency for Healthcare Research and Quality (AHRQ) standards, marking the risk of bias as unclear, high, moderate or low. Cochrane’s Q and I2 were used to indicate the heterogeneity between studies, and a funnel plot was applied to examine the risk of publication bias. A random-effects model (DerSimonian and Laird method) was used to calculate pooled odds ratios (ORs) for primary (i.e. AKI) and secondary outcomes (i.e. dialysis, death) in CM–exposed and unexposed cohorts [21]. Additional meta-regression analysis was performed to determine heterogeneity by patient-related factors (e.g. age, gender, comorbidities).
All analyses were performed using Comprehensive Meta-Analysis (CMA, Version 2.2.064) and R 4.0.2.
Results
The initial literature search identified 297 articles fulfilling our inclusion criteria. After performing full-text article workups, 26 studies were initially declared eligible for inclusion. In the course of our statistical analysis, we detected a high degree of overlapping samples in the studies by Davenport et al and McDonald et al, respectively. In order to address single author bias and increased subject weighting, we decided to remove all duplicates and consider only one study of each author. Based on the largest sample size, the studies from Davenport et al from 2013 [22] and McDonald et al from 2013 [23] were chosen for meta-analysis. As both studies lacked further subdivision into eGFR groups, the 2013 study by Davenport et al [24] and 2015 article by McDonald et al [25] were chosen for the analysis of cohorts with eGFR less than or equal to 30 mL/min/1.73 m2.
Ultimately, 21 studies were selected for final data extraction and analysis, six of which consisted of general population cohorts [22,23,24,25,26,27] (Table 1). Two studies focused on critically ill [28, 29] and two on pediatric patients [30, 31]. Four studies consisted of patients admitted via emergency department [32,33,34,35]; one study examined nephrotic syndrome patients [36]; two focused on patients with chronic kidney disease (CKD) [37, 38]; and two on patients hospitalized with AKI [39, 40]. Two groups studied septic [38, 41] and one cancer patients [42]. AKI was defined by RIFLE criteria in 1 study; by AKIN in 11 studies; by KDIGO criteria in 6 studies, and by CIN criteria in 3 studies. If studies applied AKIN and CIN criteria to define AKI, the 2007 definition by AKIN was preferred over CIN. Each study comprised two cohorts that were assigned by PSM. Here, 17 studies (80%) applied a 1:1 matching ratio. Low-osmolar contrast agents were administered in 14 studies (70%), iso-osmolar CM in one study (5%) and a combination of both in five studies (25%). One study (5%) did not specify the type of CM used. The use of high-osmolar CM was declared by none.
Effects of contrast media administration on kidney function
Overall, 7425 AKI events were detected in 60,367 patients with CM exposure and 7346 events in 51,980 controls (Table 2). There was a tendency towards lower odds of AKI in CM–exposed cohorts compared with unexposed controls (OR 0.97 [0.85; 1.11], p = 0.64) (Fig. 2). Substantial heterogeneity was detected (I2 = 90.1%, Q = 40.74, p ≤ 0.0001).
Since the risk for AKI after CM exposure is presumably higher among patients with impaired kidney function, we performed additional subgroup analysis by aggregating data from patients with eGFR ≤ 30 mL/min/1.73 m2 (Fig. 3). Here, we detected a significantly higher risk of AKI in CM–exposed patients compared with unexposed controls (OR: 1.68 [1.29; 2.19], p = 0.0001; 55; I2 = 42%, Q = 10.3, p = 0.1125) (Fig. 3). Notably, the absolute risk increase in CM–exposed patients with eGFR ≤ 30 mL/min/1.73 m2 remains rather low (334/1757, 19% vs. 863/5698, 15%) with an absolute risk increase of 4%.
Meta-regression analysis
To further explore possible origins of heterogeneity, we performed meta-regression analyses for 7 relevant covariates (Table 3). Here, a larger proportion of patients with hypertension was associated with higher odds for AKI after CM exposure (p = 0.03) (Fig. 4). Other clinically plausible variables related to the use of CM showed no significant influence on AKI rates. Therefore, hypertension and eGFR less than or equal to 30 mL/min/1.73 m2 likely are conditions accounting for the observed heterogeneity (Figs. 3 and 4).
Effects of contrast media on dialysis and mortality
A total of 1517 patients from 11 studies required renal replacement therapy after CM exposure, including 729 cases from the CM–exposed cohorts (n = 18,493) and 788 from the control groups (n = 11,202). No significant difference in the rate of dialysis was observed between CM exposed and controls (OR: 0.97 [0.75; 1.25]; p = 0.81) (Table 2).
Further, 3400 deaths were reported in 7 studies (n = 10,312), including 1649 cases among 8030 CM–exposed patients (13%) and 1751 fatal events in 2282 controls (13%). No significant difference in mortality was detected between the CM groups and controls (OR: 0.94 [0.86; 1.03]; p = 0.18) (Table 2).
Serum creatinine measurements for AKI diagnosis
Our selected studies applied different observation periods to diagnose AKI after CT imaging. Here, SCr levels were acquired within 24 h [22, 24, 29], 48 h [22, 24, 30, 31], 72 h [22, 24, 26, 42] or 96 h after the index CT scan [28, 37]. In three studies, the window for post-contrast SCr measurement reached from 48 to 72 h [33, 34, 41], one of which added a second time point between 48 h and 1 week. One group measured the SCr levels for 1 month after index CT [40], while one study refrained from defining the time period [39].
The frequency of SCr measurements following contrast-based imaging differed markedly among our studies. While three groups obtained only one SCr level after imaging [32, 33, 41], two studies measured at least three early SCr values in each 24-h period for the first 72 h after index CT [22, 24]. Eight groups reported more than one SCr measurement without disclosing the exact number [24, 26, 32, 39, 43,44,45,46]. For the remaining studies, the number of measurements was not disclosed [23, 25, 27, 30, 31, 34, 35, 38].
Risk of bias within studies
The Newcastle–Ottawa Scale indicated a low risk of bias for all studies according to the AHRQ standard (Table 4). The funnel plot (Suppl. Fig. 1) revealed no evidence of relevant publication bias. Because we hypothesized that differences in propensity score–matching methods might introduce between-study heterogeneity, an additional meta-regression analysis was performed to verify the adequacy of matching procedures (Suppl. Fig. 2). Here, we found that the inclusion of more variables in the matching model (range: 3 to 42) was associated with a greater tendency towards similar incidences of AKI between exposed and unexposed groups (p = 0.093).
Discussion
For years, the literature has been shaped by the assumption that post-contrast AKI is attributable to the iodinated CM itself rather than preexisting nephrotoxic risk factors. Building on prior studies, we sought to facilitate clinical decision-making and prevent both over- and underestimation of AKI risk during CT examination. Risk overestimation might deprive patients of clinical benefits of contrast-enhanced imaging out of fear of causing AKI. However, an underestimation could expose patients to preventable nephrotoxic insults with high potential for adverse outcomes.
We performed a systematic review and meta-analysis of 21 cohort studies utilizing a propensity-matched multivariate model, in order to isolate the role of CM exposure on the incidence of post-contrast AKI. In line with the growing body of literature, we found no evidence from state-of-the-art cohort studies for an increased risk for AKI, dialysis or mortality after single administration of CM during CT scan in eGFR groups above 45 mL/min/1.73 m2. These results appear robust, even in subgroups with chronic and critical illness. However, our analysis revealed an increased risk of AKI in patients with eGFR of less than or equal to 30 mL/min/1.73 m2 and hypertensive disease.
Previous studies on CM nephrotoxicity were limited either by a lack of control groups or by absence of adjustments for predisposing risk factors [43, 44, 47, 48]. The study by Moos et al [48] has stood out by including four studies with unexposed controls (out of a total of 41 studies). Retrospective observational studies followed, showing similar rates of AKI following CT examination regardless of CM administration [45, 46]. Moreover, a substantial number of patients without CM exposure displayed changes in SCr levels that would have met the criteria for CIN, had they undergone CM administration [45]. This emphasized the need for a proper comparator arm. Observational controlled studies followed, presenting similar rates of AKI between CM–exposed patients and unexposed controls. Currently, PSM protocols are employed to adjust for patient-related factors (e.g. age, sex) and various underlying comorbidities amongst study cohorts, thereby approximating a randomized distribution. Our study further expands upon contemporary meta-analyses that either partly [49] or entirely [50] lacked matched controls, or featured considerably fewer studies [19].
Our findings reverberate the conflicting data in the adult literature regarding renal risks after intravenous CM administration and prompt a differential analysis for patients with high disease burden.
Assessing a broad range of comorbidities, no other association with higher AKI risk was found. This is particularly noteworthy considering that our study populations featured critically [28, 29, 34, 35] and chronically ill patients [25, 37, 39]. In daily clinical practice, these patients are most likely to experience exclusion from CM–enhanced procedures out of fear of causing contrast-induced AKI. Our findings do not support the clinical avoidance of CM where otherwise indicated. Similarly, van der Molen et al recently demonstrated no need for emergency haemodialysis after administration of iodine-based CM in patients with dialysis-requiring CKD [51]. The observed shift in the medical literature may be explained by adjustments in CM osmolality and administered volumes in recent years.
Similar to other authors, we observed a trend towards lower risk for renal impairment after CM exposure [32, 33]. Puchol et al explained this with the hydration occurring in the course of administering the CM volume, and its subsequent osmotic diuretic effects [32]. Since CM is not nephroprotective, we assume the presence of factors affecting the AKI incidence. These are not easily measurable and, as it seems, not entirely rectified by PSM. Investigators who applied PSM models have reported similar results [52]. Studies cannot consider factors that conceivably bias the decision to administer CM in the first place. Therefore, it remains crucial to consider the possible impact of these variables before and after PSM, in order to avoid misleading inferences of causality. Selection bias may also cause the higher number of AKI among controls (i.e. unenhanced CT). This arises when presumed high-risk patients are excluded from CM exposure under the assumption that CM causes AKI, which precipitates a less healthy control cohort. Likewise, discrepancies in matching methodologies or small sample sizes may contribute to this finding [22]. Conceivably, patients with contrast-enhanced imaging might receive a better fluid management as part of the CM administration protocol.
Our study displays various methodological strengths. We focused on cohort studies originally designed to compare the nephrotoxicity of CM–enhanced CT with unenhanced CT examinations. By restricting the analysis to studies that applied PSM, we further narrowed limitations of inherent biases in observational study designs [53]. Our findings affirm that PSM does not account for all influencing factors and that all outcomes require careful interpretation. However, since randomized controlled trials evaluating post-contrast AKI remain unlikely for ethical reasons, our findings summarize the best available evidence in absence of randomization. By excluding all studies that lacked controls, we further enhanced the rigor of our analysis. AKI diagnosis was made based on internationally recognized guidelines, the anticipated primary event of interest was documented and standardization across all studies was established in terms of design (i.e. observational cohort study), intervention (i.e. CT scan) and primary outcome (i.e. AKI).
To the best of our knowledge, we provide the first and most extensive study that systematically assesses the renal risks after CT examination attributable to CM after controlling for demographic variables. Despite this, we note limitations in our study, which deserve mention. Our data relies on retrospective cohort studies with limited numbers of participants. One study did not disclose the osmolality of CM used. However, based on the recency of publication (i.e. 2019), the use of high-osmolar CM for CT is quite unlikely. Further, neither fluid administration during and after CT nor nephrotoxic medications were consistently documented throughout the studies. This would have been preferable, as hydration is known to reduce the risk of post-contrast renal impairment [51]. With regard to total volumes of injected CM, only weight-adjusted ranges were provided (n = 15). Notably, none of the groups described the flow rate of intravenous CM administration and only eight disclosed CM concentrations. We strongly recommend the disclosure of all periprocedural circumstances for accurate risk estimation and effective periprocedural management.
This also applies to post-contrast serial measurements of SCr in clinical settings. Since AKI is not necessarily associated with permanent changes in renal function, consistent SCr measurement protocols following CM administration would be of great value to improve the diagnostic algorithm in suspected AKI. Diagnostic standardization with longer observation periods may help differentiate between subclinical renal damage and potentially reversible background fluctuations of SCr. Lastly, the majority of studies failed to report AKI stages, which would have been beneficial to understand the severity of kidney injury and show the risk of progression to higher AKI stages.
Currently, no adjunctive medication can effectively prevent or treat post-contrast AKI. Therefore, it remains crucial to anticipate and obviate post-contrast renal decline with comprehensive risk prediction scores and preprocedural volume expansion, even in emergencies and time-sensitive conditions [54]. The clinical practice of withholding CM–enhanced imaging for concern of CI-AKI appears not to be justified. However, despite the low incremental risk, caution remains warranted in individuals with hypertension or eGFR less than or equal to 30 mL/min/1.73 m2.
Abbreviations
- AKI:
-
Acute kidney injury
- AKIN:
-
Acute Kidney Injury Network
- CA-AKI:
-
Contrast-associated acute kidney injury
- CI-AKI:
-
Contrast-induced acute kidney injury
- CIN:
-
Contrast-induced nephropathy
- CM:
-
Contrast media
- SCr:
-
Serum creatinine
- CT:
-
Computed tomography
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- RIFLE:
-
Risk, injury, failure, loss of kidney function, and end-stage kidney disease
- 95%CI:
-
95% confidence interval
- PS:
-
Propensity score
- PSM:
-
Propensity score matching
- CKD:
-
Chronic kidney disease
References
Bartels ED, Brun GC, Gammeltoft A, Gjørup PA (1954) Acute anuria following intravenous pyelography in a patient with myelomatosis. Acta Med Scand 150(4):297–302
Goldfarb S, McCullough PA, McDermott J, Gay SB (2009) Contrast-induced acute kidney injury: specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc 84:170–179
Mohammed NMA, Mahfouz A, Achkar K, Rafie IM, Hajar R (2013) Contrast-induced nephropathy. Heart Views 14:106–116
Castini D, Lucreziotti S, Bosotti L et al (2010) Prevention of contrast-induced nephropathy: a single center randomized study. Clin Cardiol 33:E63–E68
Mehran R, Nikolsky E (2006) Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl 100:S11–S15
Spargias K, Alexopoulos E, Kyrzopoulos S et al (2004) Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 110:2837–2842
Ahmed K, McVeigh T, Cerneviciute R et al (2018) Effectiveness of contrast-associated acute kidney injury prevention methods; a systematic review and network meta-analysis. BMC Nephrol 19(1):323
Van der Molen AJ, Reimer P, Dekkers IA et al (2018) Post-contrast acute kidney injury - Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors: recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 28(7):2845–2855
Levy EM, Viscoli CM, Horwitz RI (1996) The effect of acute renal failure on mortality. A cohort analysis. JAMA 275:1489–1494
Mitchell AM, Jones AE, Tumlin JA, Kline JA (2010) Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol 5:4–9
Benedetto U, Head SJ, Angelini GD, Blackstone EH (2018) Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg 53:1112–1117
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424
Mehta RL, Kellum JA, Shah SV et al (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120(4):c179–c184
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup (2004) Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Luk L, Steinman J, Newhouse JH (2017) Intravenous contrast-induced nephropathy—the rise and fall of a threatening idea. Adv Chronic Kidney Dis 24:169–175
Nyman U, Aspelin P, Jakobsen J, Björk J (2015) Controversies in contrast material−induced acute kidney injury: propensity score matching of patients with different dose/absolute glomerular filtration rate ratios. Radiology 277:633–637
Ehrmann S, Quartin A, Hobbs BP et al (2017) Contrast-associated acute kidney injury in the critically ill: systematic review and Bayesian meta-analysis. Intensive Care Med 43(6):785–794
Rudnick MR, Leonberg-Yoo AK, Lit HI (2020) The controversy of contrast-induced nephropathy with intravenous contrast: what is the risk? Am J Kidney Dis 75:105–113
Wells GA, Shea B, O’Connell D, et al (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Psychology. Available via http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf. Accessed August 1 2021
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Davenport MS, Khalatbari S, Dillman JR, Cohan RH, Caoili EM, Ellis JH (2013) Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology 267(1):94–105
McDonald RJ, McDonald JS, Bida JP et al (2013) Intravenous contrast material–induced nephropathy: causal or coincident phenomenon? Radiology 267:106–118
Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH (2013) Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 268:719–728
McDonald JS, McDonald RJ, Lieske JC et al (2015) Risk of acute kidney injury, dialysis, and mortality in patients with chronic kidney disease after intravenous contrast material exposure. Mayo Clin Proc 90:1046–1053
Elias A, Aronson D (2021) Risk of acute kidney injury after intravenous contrast media administration in patients with suspected pulmonary embolism: a propensity-matched study. Thromb Haemost 121(6):800–807
Gorelik Y, Bloch-Isenberg N, Heyman SN, Khamaisi M (2021) Renal functional recovery confounding the assessment of contrast nephropathy: propensity score analysis. Am J Nephrol 52(1):76–83
Ehrmann S, Badin J, Savath L et al (2013) Acute kidney injury in the critically ill: is iodinated contrast medium really harmful? Crit Care Med 41:1017–1026
Williams LMS, Walker GR, Loewenherz JW, Gidel LT (2020) Association of contrast and acute kidney injury in the critically ill: a propensity matched study. Chest 157:866–876
Guo S, Bai L, Tong Y et al (2021) Contrast media exposure in the perioperative period confers no additional risk of acute kidney injury in infants and young children undergoing cardiac surgery with cardiopulmonary bypass. Pediatr Nephrol 36(8):2485–2491
Gilligan LA, Davenport MS, Trout AT et al (2020) Risk of acute kidney injury following contrast-enhanced CT in hospitalized pediatric patients: a propensity score analysis. Radiology 294:548–556
Puchol F, García M, Navarro F, Rodrigo S, Pérez B, López T (2019) The administration of contrast media: Is there a risk of acute kidney injury? Radiología (Engl Ed) 61:306–314
Hinson JS, Ehrmann MR, Fine DM et al (2016) Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med 69:577–586
Su TH, Hsieh CH, Chan YL et al (2021) Intravenous CT contrast media and acute kidney injury: a multicenter emergency department-based study. Radiology 301(3):571–581
Kene M, Arasu VA, Mahapatra AK, Huang J, Reed ME (2021) Acute kidney injury after CT in emergency patients with chronic kidney disease: a propensity score-matched analysis. West J Emerg Med 22(3):614–622
Tao SM, Kong X, Schoepf UJ et al (2018) Acute kidney injury in patients with nephrotic syndrome undergoing contrast-enhanced CT for suspected venous thromboembolism: a propensity score-matched retrospective cohort study. Eur Radiol 28:1585–1593
Chaudhury P, Armanyous S, Harb SC et al (2019) Intra-arterial versus intravenous contrast and renal injury in chronic kidney disease: a propensity-matched analysis. Nephron 141:31–40
Ellis JH, Khalatbari S, Yosef M, Cohan RH, Davenport MS (2019) Influence of clinical factors on risk of contrast-induced nephrotoxicity from IV iodinated low-osmolality contrast material in patients with a low estimated glomerular filtration rate. AJR Am J Roentgenol 213:W188–W193
Goto Y, Koyama K, Katayama S et al (2019) Influence of contrast media on renal function and outcomes in patients with sepsis-associated acute kidney injury: a propensity-matched cohort study. Crit Care 23:249
Yan P, Zhang NY, Luo XQ et al (2022) Is intravenous iodinated contrast medium administration really harmful in hospitalized acute kidney injury patients: a propensity score-matched study. Eur Radiol 32(2):1163–1172
Hinson JS, Al Jalbout N, Ehrmann MR, Klein EY (2019) Acute kidney injury following contrast media administration in the septic patient: a retrospective propensity-matched analysis. J Crit Care 51:111–116
Latcha S, Plodkowski AJ, Zheng J, Jaimes EA (2019) Rate and risk factors for AKI after CT scans in a cancer cohort. Clin Nephrol 91:147–154
Biondi-Zoccai G, Lotrionte M, Thomsen HS et al (2014) Nephropathy after administration of iso-osmolar and low-osmolar contrast media: evidence from a network meta-analysis. Int J Cardiol 172(2):375–380
Song W, Zhang T, Pu J, Shen L, He B (2014) Incidence and risk of developing contrast-induced acute kidney injury following intravascular contrast administration in elderly patients. Clin Interv Aging 9:85–93
Newhouse JH, Kho D, Rao QA, Starren J (2008) Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol 191(2):376–382
Bruce RJ, Djamali A, Shinki K, Michel SJ, Fine JP, Pozniak MA (2009) Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol 192(3):711–718
Dong M, Jiao Z, Liu T, Guo F, Li G (2012) Effect of administration route on the renal safety of contrast agents: a meta-analysis of randomized controlled trials. J Nephrol 25(3):290–301
Moos SI, van Vemde DN, Stoker J, Bipat S (2013) Contrast induced nephropathy in patients undergoing intravenous (IV) contrast enhanced computed tomography (CECT) and the relationship with risk factors: a meta-analysis. Eur J Radiol 82(9):e387–e399
Aycock RD, Westafer LM, Boxen JL, Majlesi N, Schoenfeld EM, Bannuru RR (2018) Acute kidney injury after computed tomography: a meta-analysis. Ann Emerg Med 71(1):44–53
McDonald JS, McDonald RJ, Comin J et al (2013) Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology 267(1):119–128
Van der Molen AJ, Reimer P, Dekkers IA et al (2018) Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients: recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 28(7):2856–2869
Wilhelm-Leen E, Montez-Rath ME, Chertow G (2017) Estimating the risk of radiocontrast-associated nephropathy. J Am Soc Nephrol 28(2):653–659
Newhouse JH, RoyChoudhury A (2013) Quantitating contrast medium-induced nephropathy: controlling the controls. Radiology 267(1):4–8
Mehran R, Aymong ED, Nikolsky E et al (2004) A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 44(7):1393–1399
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Bernhard Magnus Wilhelm Schmidt, MD, SM/M.Sc.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was not required, because this work is a systematic review and meta-analysis.
Ethical approval
Institutional Review Board approval was not required, because this work is a systematic review and meta-analysis.
Methodology
• retrospective
• not applicable
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 627 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obed, M., Gabriel, M.M., Dumann, E. et al. Risk of acute kidney injury after contrast-enhanced computerized tomography: a systematic review and meta-analysis of 21 propensity score–matched cohort studies. Eur Radiol 32, 8432–8442 (2022). https://doi.org/10.1007/s00330-022-08916-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08916-y