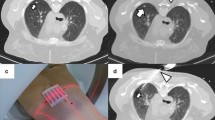
Abstract
Objective
To compare temporal changes of ablation zones and lymph nodes following lung microwave ablation (MWA) and cryoablation.
Methods
This retrospective cohort study compared lung ablation zones and thoracic lymph nodes following MWA and cryoablation performed 2006–2020. In the ablation zone cohort, ablation zone volumes were measured on serial CT for 12 months. In the lymph node cohort, the sum of bidimensional products of lymph node diameters was measured before (baseline) and up to 6 months following ablation. Cumulative incidence curves estimated the time to 75% ablation zone reduction and linear mixed-effects regression models compared the temporal distribution of ablation zones and lymph node sizes between modalities.
Results
Ablation zones of 59 tumors treated in 45 sessions (16 MWA, 29 cryoablation) in 36 patients were evaluated. Differences in the time to 75% volume reduction between modalities were not detected. Following MWA, half of the ablation zones required an estimated time of 340 days to achieve a 75% volume reduction compared to 214 days following cryoablation (p = .30). Thoracic lymph node sizes after 33 sessions (13 MWA, 20 cryoablation) differed between modalities (baseline–32 days, p = .01; 32–123 days, p = .001). Following MWA, lymph nodes increased on average by 38 mm2 (95%CI, 5.0–70.7; p = .02) from baseline to 32 days, followed by an estimated decrease of 50 mm2 (32–123 days; p = .001). Following cryoablation, changes in lymph nodes were not detected (baseline–32 days, p = .33).
Conclusion
The rate of ablation zone volume reduction did not differ between MWA and cryoablation. Thoracic lymph nodes enlarged transiently after MWA but not after cryoablation.
Key Points
• Contrary to current belief, the rate of lung ablation zone volume reduction did not differ between microwave and cryoablation.
• Transient enlargement of thoracic lymph nodes after microwave ablation was not associated with regional tumor spread and decreased within six months following ablation.
• No significant thoracic lymph node enlargement was observed following cryoablation.






Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- ECOG:
-
Eastern Cooperative Oncology Group
- HU:
-
Hounsfield units
- IQR:
-
Interquartile range
- mRECIST:
-
Modified Response Evaluation Criteria in Solid Tumors
- MWA:
-
Microwave ablation
- NE:
-
Not estimable
- RFA:
-
Radiofrequency ablation
References
de Baere T, Tselikas L, Woodrum D et al (2015) Evaluating cryoablation of metastatic lung tumors in patients--safety and efficacy: the ECLIPSE trial--interim analysis at 1 year. J Thorac Oncol 10:1468–1474
Moore W, Talati R, Bhattacharji P, Bilfinger T (2015) Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol 26:312–319
Iezzi R, Cioni R, Basile D et al (2020) Standardizing percutaneous microwave ablation in the treatment of lung tumors: a prospective multicenter trial (MALT study). Eur Radiol. https://doi.org/10.1007/s00330-020-07299-2
Palussiere J, Catena V, Buy X (2017) Percutaneous thermal ablation of lung tumors - Radiofrequency, microwave and cryotherapy: where are we going? Diagn Interv Imaging 98:619–625
Leppelmann KS, Levesque VM, Bunck AC et al (2021) Outcomes following percutaneous microwave and cryoablation of lung metastases from adenoid cystic carcinoma of the head and neck: a bi-institutional retrospective cohort study. Ann Surg Oncol 28:5829–5839
Fintelmann FJ, Braun P, Mirzan SH et al (2020) Percutaneous cryoablation: safety and efficacy for pain palliation of metastases to pleura and chest wall. J Vasc Interv Radiol 31:294–300
Dupuy DE (2011) Image-guided thermal ablation of lung malignancies. Radiology 260:633–655
Ito N, Nakatsuka S, Inoue M et al (2012) Computed tomographic appearance of lung tumors treated with percutaneous cryoablation. J Vasc Interv Radiol 23:1043–1052
Chheang S, Abtin F, Guteirrez A, Genshaft S, Suh R (2013) Imaging features following thermal ablation of lung malignancies. Semin Intervent Radiol 30:157–168
Abtin F, De Baere T, Dupuy DE et al (2019) Updates on current role and practice of lung ablation. J Thorac Imaging 34:266–277
Prud'homme C, Deschamps F, Moulin B et al (2019) Image-guided lung metastasis ablation: a literature review. Int J Hyperthermia 36:37–45
Wei Z, Ye X, Yang X et al (2015) Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol 38:135–142
Herrera LJ, Fernando HC, Perry Y et al (2003) Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg 125:929–937
Lyons GR, Askin G, Pua BB (2018) Clinical outcomes after pulmonary cryoablation with the use of a triple freeze protocol. J Vasc Interv Radiol 29:714–721
Vogl TJ, Naguib NN, Gruber-Rouh T, Koitka K, Lehnert T, Nour-Eldin N-EA (2011) Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 261:643–651
Al-Hakim RA, Abtin FG, Genshaft SJ, Kutay E, Suh RD (2016) Defining new metrics in microwave ablation of pulmonary tumors: ablation work and ablation resistance score. J Vasc Interv Radiol 27:1380–1386
Deandreis D, Leboulleux S, Dromain C et al (2011) Role of FDG PET/CT and chest CT in the follow-up of lung lesions treated with radiofrequency ablation. Radiology 258:270–276
Abtin FG, Eradat J, Gutierrez AJ, Lee C, Fishbein MC, Suh RD (2012) Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics 32:947–969
Sharma A, Digumarthy SR, Kalra MK, Lanuti M, Shepard JA (2010) Reversible locoregional lymph node enlargement after radiofrequency ablation of lung tumors. AJR Am J Roentgenol 194:1250–1256
Wolf FJ, Grand DJ, Machan JT, DiPetrillo TA, Mayo-Smith WW, Dupuy DE (2008) Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 247:871–879
Wrobel MM, Bourgouin PP, Abrishami Kashani M et al (2021) Active versus passive thaw following percutaneous cryoablation of pulmonary tumors: effect on incidence, grade, and onset of hemoptysis. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.21.25872
Bourgouin PP, Wrobel MM, Mercaldo ND et al (2021) Comparison of percutaneous image-guided microwave ablation and cryoablation for sarcoma lung metastases: a 10-year experience. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.21.26551:1-11
Ahmed M, Technology Assessment Committee of the Society of Interventional R (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update: supplement to the consensus document. J Vasc Interv Radiol 25:1706–1708
Hanley JA, Negassa A, Edwardes MD, Forrester JE (2003) Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 157:364–375
Puijk RS, Ahmed M, Adam A et al (2021) Consensus guidelines for the definition of time-to-event end points in image-guided tumor ablation: results of the SIO and DATECAN initiative. Radiology. https://doi.org/10.1148/radiol.2021203715:203715
Zhao X, Zhao Q, Sun J, Kim JS (2008) Generalized log-rank tests for partly interval-censored failure time data. Biom J 50:375–385
Luke SG (2017) Evaluating significance in linear mixed-effects models in R. Behav Res Methods 49:1494–1502
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Das JP, Barry C, Schoder H, Camacho JC, Ginsberg MS, Halpenny DF (2021) Imaging following thermal ablation of early lung cancers: expected post-treatment findings and tumour recurrence. Clin Radiol. https://doi.org/10.1016/j.crad.2021.07.009
Ahrar K, Tam AL, Kuban JD, Wu CC (2021) Imaging of the thorax after percutaneous thermal ablation of lung malignancies. Clin Radiol. https://doi.org/10.1016/j.crad.2021.07.007
Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z (2005) Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology 235:289–298
Riquet M, Hidden G, Debesse B (1989) Direct lymphatic drainage of lung segments to the mediastinal nodes. J Thoracic Cardiovasc Surg 97:623–632
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Florian J. Fintelmann.
Conflict of interest
Florian J. Fintelmann received salary support from the American Roentgen Ray Society and the William M. Wood Foundation during the study period, as well as research support from Boston Scientific. Amita Sharma reports research support from Hummingbird Diagnostics Inc. for unrelated work. The other authors have no relevant conflicts of interest to report.
Statistics and biometry
Nathaniel D. Mercaldo, PhD, is a biostatistician in the Department of Radiology at Massachusetts General Hospital and performed the statistical analysis for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
The current study overlaps with two previously published reports (references #21, # 22) in that the clinical outcomes of 28 patients in the current study were previously described. The current study differs significantly because it focuses only on imaging features following percutaneous lung ablation, regardless of clinical outcomes.
Methodology
• retrospective
• observational
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Figure 1
(PNG 282 kb)
ESM 1
(DOCX 33 kb)
Supplementary Table 1
(DOCX 21 kb)
Supplementary Table 2
(DOCX 17 kb)
Supplementary Table 3
(DOCX 17 kb)
Supplementary Table 4
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Wrobel, M.M., Cahalane, A.M., Pachamanova, D. et al. Comparison of expected imaging findings following percutaneous microwave and cryoablation of pulmonary tumors: ablation zones and thoracic lymph nodes. Eur Radiol 32, 8171–8181 (2022). https://doi.org/10.1007/s00330-022-08905-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08905-1