Abstract
Objectives
This study aimed to evaluate the association between visual emphysema and the presence of lung nodules, and Lung-RADS category with low-dose CT (LDCT).
Methods
Baseline LDCT scans of 1162 participants from a lung cancer screening study (Nelcin-B3) performed in a Chinese general population were included. The presence, subtypes, and severity of emphysema (at least trace) were visually assessed by one radiologist. The presence, size, and classification of non-calcified lung nodules (≥ 30 mm3) and Lung-RADS category were independently assessed by another two radiologists. Multivariable logistic regression and stratified analyses were performed to estimate the association between emphysema and lung nodules, Lung-RADS category, after adjusting for age, sex, BMI, smoking status, pack-years, and passive smoking.
Results
Emphysema and lung nodules were observed in 674 (58.0%) and 424 (36.5%) participants, respectively. Participants with emphysema had a 71% increased risk of having lung nodules (adjusted odds ratios, aOR: 1.71, 95% CI: 1.26–2.31) and 70% increased risk of positive Lung-RADS category (aOR: 1.70, 95% CI: 1.09–2.66) than those without emphysema. Participants with paraseptal emphysema (n = 47, 4.0%) were at a higher risk for lung nodules than those with centrilobular emphysema (CLE) (aOR: 2.43, 95% CI: 1.32–4.50 and aOR: 1.60, 95% CI: 1.23–2.09, respectively). Only CLE was associated with positive Lung-RADS category (p = 0.02). CLE severity was related to a higher risk of lung nodules (ranges aOR: 1.44–2.61, overall p < 0.01).
Conclusion
In a Chinese general population, visual emphysema based on LDCT is independently related to the presence of lung nodules (≥ 30 mm3) and specifically CLE subtype is related to positive Lung-RADS category. The risk of lung nodules increases with CLE severity.
Key Points
• Participants with emphysema had an increased risk of having lung nodules, especially smokers.
• Participants with PSE were at a higher risk for lung nodules than those with CLE, but nodules in participants with CLE had a higher risk of positive Lung-RADS category.
• The risk of lung nodules increases with CLE severity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the primary cause of cancer death worldwide [1]. It ranked sixth among the top 10 leading causes of death in 2019, rising from 1.2 million to 1.8 million cases since 2000. In China, which is currently the most populous country in the world, lung cancer is the malignancy with the highest incidence and the main cause of cancer-related mortality [2]. Even though the treatment of lung cancer is gradually improving, the 5-year survival rate was still as low as 16.5% in 2012–2015 [3]. The increasing lung cancer burden in China combined with poor prognosis is a challenge for cancer prevention. LDCT screening among high-risk individuals could lead to a 20–33% lung cancer-specific mortality reduction [4]. As a consequence, LDCT screening has been introduced and is now recommended as a strategy for the early detection of lung cancer worldwide [5].
With the progress of higher spatial resolution of CT scanners combined with more advanced postprocessing software, the prevalence of lung nodules in lung cancer screening may vary from 21 to 86% depending on acquisition protocol, the population included, and guidelines used during 2006–2018 [6]. Although the majority of detected lung nodules are benign, lung cancer rate in participants with non-calcified pulmonary baseline nodules ranges from 2 to 11% [7, 8]. About 3–4% of the participants with a non-calcified nodule at baseline will develop lung cancer within the following 2–5 years [9]. The risk factors for lung cancer such as size, age, and smoking have been established [10], but far less is known about risk factors for the presence of lung nodules and its malignant risk. This may be critical because lung nodules are the early manifestations of lung cancer.
Emphysema is a pathologic condition with enlargement of air spaces in the terminal bronchioles. It is one of the predictive risk factors for neoplastic transformation of the lining epithelium [11]. There are numerous studies on risk factors involving emphysema as a risk factor for lung cancer [12, 13]. Some studies on lung cancer screening suggest that 48–58% of the participants with emphysema also have lung nodules [14], which could lead to a higher lung cancer mortality because of increased susceptibility to biological damage [15]. Although emphysema and the presence of lung nodules have shared risk factors, such as age and smoking, it is not clear whether emphysema is independently associated with the presence of lung nodules. In previous studies, this could not be investigated since most studies only included a high-risk (smoking) population. However, identifying new risk factors for lung nodules or the malignant risk is important in the future to optimize nodule management of incidentally detected lung nodules in the general population.
For that, the present study aimed to evaluate the association between the presence, subtypes, and severity of visual emphysema, and the presence of lung nodules, as assessed by LDCT in a general Chinese population. The association between emphysema and malignant risk (Lung-RADS category) of lung nodules was also evaluated.
Materials and methods
Participants
The here presented study used data from participants that were included in the Nelcin-B3 study. In the Nelcin-B3 study, a general Chinese population was recruited without inclusion criteria on smoking and pack-years to identify risk factors for the “Big 3” diseases (lung cancer, cardiovascular disease, and COPD), and reference values based on LDCT [16]. The study was approved by the Ethics Committee of Biomedicine Research of Second Military Medical University (registration number: NCT03992833). At the Radiology Department of Tianjin Medical University Cancer Institute and Hospital (TJMUCIH), 4000 participants from the general population, being Tianjin residents for 3 years or longer 3 years, aged between 40 and 74 years, without any history of cancer, were invited for LDCT lung cancer screening. The current analysis focused on a consecutive series of the participants included in the Nelcin-B3 study that underwent LDCT between May and October 2017 (see Fig. 1). Participants were excluded if they had incomplete data or pneumothorax. All participants signed the informed consent, and a structured face-to-face interview was conducted by trained interviewers to gather demographic information (age, sex, ethnicity, smoking status, pack-years, passive smoking, BMI). Passive smoking was defined as an indoor environment where participants inhale smoke produced by others ≥ 1 day a week for ≥ 15 min. About the pack-years, zero was scored for never smoker.
CT scan acquisition and interpretation
The CT chest examinations were performed with a 64-detector row CT system (Somaton Definition AS 64, Siemens) using a low-dose technique without the use of a contrast agent. Details of the technical parameters used in the CT scan protocol were as follows: 120 kVp, 35 mAs, pitch of 1.0, reconstruction kernels D45F (emphysema assessment), and B80F (lung nodules assessment) were applied to reconstruct images at 1.0/0.7 mm thickness and increment. All the participants were scanned in the supine position with head forward. The CT scans were obtained at deep inspiration with the breath-holding of subjects under the above technical setting.
Lung nodule assessment
After prospective collection of LDCT scans, the detection and features of suspected lung nodules were retrospectively assessed by one junior chest radiologist (Y.F.M. with 4 years of experience) and checked by another senior chest radiologist (D.M. with 10 years of experience). The maximum intensity projection (MIP) technique was used to detect lung nodules under the D45F kernel with 10-mm slice thickness. Three-dimensional segmentation software (MM Oncology Syngo.via., version VB30A, Siemens) was used to automatically measure the volume of each detected nodule under the B80F kernel. All CT scans used for lung nodule detection were read at both lung window (window center: −500 HU, window width: 1200 HU) and mediastinal window (window center: 35 HU, window width: 320 HU). Nodules were scored and classified as solid, part-solid, and non-solid nodules (pure ground-glass nodules). Nodule location was classified as upper lobe (right middle, left, or right upper lobe) or lower lobe (left or right lower lobe). Included in the here presented analysis were all non-calcified nodules with volume ≥ 30 mm3 [16,17,18]. LDCT scan findings were assessed using Lung-RADS category (1, 2, 3, and 4) [19].
Visual emphysema assessment
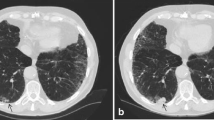
The visual emphysema assessment was performed using the Minimum Intensity Projection (Minip) technique with 10-mm thickness in the lung window setting (window center: −850 HU, window width: 400 HU) [20] and Multiplanar Reconstruction (MPR) technique with 1-mm thickness in window setting (window center: −750 HU, window width: 700 HU) [21] all based on the D45F reconstruction kernel CT images with the same software used for nodule assessment. All images were visually assessed by one radiologist (X.F.Y. with 5 years of experience) using a standard protocol based on validated criteria created by the Fleischner Society [22]. Interobserver agreement was determined based on 100 randomly selected cases assessed by a second radiologist (Z.H.Y. with 2 years of experience).
Emphysema was scored as well-defined or ill-defined low attenuation or lucencies following the Fleischner criteria [22]. If present (at least trace), emphysema was further categorized as one of the three predominant subtypes of emphysema (centrilobular[CLE], paraseptal [PSE], and panlobular). The severity of CLE was categorized into trace (< 0.5%), mild (0.5–5%), moderate (> 5%), confluent and advanced destructive (confluent-ADE) according to the morphology and most severe percentage of lucency in a lung zone.
Statistical methods
Kappa statistics for the presence of emphysema and weighted kappa coefficients for CLE and PSE severity were calculated to assess interobserver agreement. Participants were classified as having no nodule versus having at least one nodule, and negative Lung-RADS (1 or 2) versus positive (3 or 4). Baseline characteristics of participants were described, overall and stratified by the presence or absence of lung nodules. To estimate the association between the baseline characteristics and the presence of lung nodules, univariate logistic regression analysis was performed to estimate odds ratios (ORs) and related 95% confidence intervals (95% CIs). To adjust for covariates, the following characteristics were selected: age, sex, smoking status, pack-years, passive smoking, and BMI. Those were based on previous literature [23, 24]. Multivariable logistic regression analysis was performed with “enter approach” to estimate aORs and related 95% CIs. In addition, an interaction term for smoking status and emphysema was included in the model. Given that analysis was significant, a stratified analysis was performed for smokers (current smokers) and non-smokers (never or former smokers). The predictors of interest were as follows: the presence of emphysema, the subtypes of emphysema, and the severity of CLE. The severity of emphysema was entered as an ordinal variable to investigate the effect in each category and as a numerical variable to test for linear trend. Mann-Whitney U testing and chi-square testing were conducted to analyze the association between emphysema and size, number, classification, and location of lung nodules. The nodule size, classification, and location were determined according to the largest nodule, and the highest Lung-RADS category was taken into account when more than one nodule was present. The number of nodules was categorized into three categories: 1 nodule, 2–3 nodules, 4 and more nodules. Statistical analysis was conducted using the SPSS 23.0 (IBM Corporation). p < 0.05 was considered a statistically significant difference.
Results
Participant characteristics
In total, 1162 participants were included in this analysis (see Fig. 1). The mean age of the participants was 61.2 ± 6.9 years, 517 (44.5%) were male, and 781 (67.2%) were never smokers (see Table 1). Of the 1162 participants, 424 (36.5%) had lung nodules and 674 (58.0%) participants had emphysema. Concerning the predominant subtypes of emphysema, the most frequent emphysema subtype was centrilobular (93%) including 58 (9.3% ) moderate, confluent, or ADE severity, followed by PSE (7.0%). There were no participants with panlobular emphysema.
Interobserver agreement
Agreement between radiologist for assessment of presence of emphysema was good (κ = 0.76 (95% CI: 0.63–0.89). Similarly, agreement was good for severity of CLE (κweighted = 0.77, 95% CI: 0.67–0.88) and PSE (κweighted=0.77, 95% CI: 0.58–0.96).
Association between participant characteristics and lung nodules presence
Participants with lung nodules were slightly older (mean 61.8 vs 60.8 years, OR: 1.02, 95% CI: 1.00–1.04), and were more frequently male (50.5% vs 49.5%, OR: 1.46, 95% CI: 1.15–1.86), compared to participants without lung nodules (see Table 1). Furthermore, participants with lung nodules had a higher prevalence of visual emphysema (66.7% vs 53.0%, OR: 1.78, 95% CI: 1.39–2.28). There were no significant differences regarding ethnicity, smoking status, pack-years, BMI, and passive smoking between participants with and without lung nodules.
Association between emphysema and lung nodules presence, Lung-RADS category
Multivariable analysis showed that participants with emphysema based on visual assessment increased the risk of lung nodules by 71% (aOR: 1.71, 95% CI: 1.26–2.31; see Table 2) compared to participants without emphysema. Stratified results showed that the higher risk in participants with emphysema was more pronounced in smokers (aOR: 2.28, 95% CI: 1.17–4.43), but was also seen in non-smokers (aOR: 1.55, 95% CI: 1.16–2.06). PSE seemed to be associated with a somewhat higher risk of lung nodules than CLE (aOR: 2.43, 95% CI: 1.32–4.50, and aOR: 1.60, 95% CI: 1.23–2.09, respectively), compared with participants without any emphysema (see Table 3). With respect to emphysema severity, the aORs for lung nodules gradually increased (aOR range: 1.44–2.61, overall p < 0.01, see Table 4) with the severity of CLE. The aOR for lung nodule was still significantly different when emphysema severity was considered a continuous variable (aOR: 1.40, 95% CI: 1.19–1.64, p for trend < 0.001).
The multivariable analysis also showed that presence of emphysema in a participant increased the risk of positive Lung-RADS category by 70% (aOR: 1.70, 95% CI: 1.09–2.66; see Table S1) compared to a participant without emphysema. When stratified by subtype of emphysema, CLE was associated with positive Lung-RADS category (aOR: 1.69, 95% CI: 1.08–2.66), whereas this was not shown for PSE (aOR: 1.83, 95% CI: 0.71–4.72).
Emphysema in relation to lung nodule characteristics
Of the 424 participants with lung nodules, 284 (67.0%, 284 of 424) had a single nodule. There was a difference in the total number of nodules between those with and without emphysema (overall p < 0.01, see Table 5). Participants with emphysema were more likely to have 2–3 nodules versus those without (31.8% vs 17.0%, respectively) (p < 0.01). Participants with emphysema had larger nodules (median 74 vs 62 mm3, p = 0.03) when compared to participants without. Most participants with nodules had solid nodules (90.8%, 385 of 424), followed by non-solid nodules (6.6%, 28 of 424) and part-solid nodules (2.6%, 11 of 424). This remained true when comparing participants with and without emphysema. In 223 (52.6%, 223 of 424) participants, nodules were located in the upper lobe. No significant difference was found when comparing participants with and without emphysema.
Discussion
In this LDCT screening study in a general Chinese population, we explored the association between the presence of emphysema and the presence of lung nodules. Participants with visual emphysema had a 71% increased risk of having at least one non-calcified lung nodule compared to those without emphysema. In particular, participants with PSE had a higher risk to have lung nodules but nodules in participants with CLE had a higher risk of positive Lung-RADS category (3 or 4). There was a severity-dependent effect; more CLE conferred a higher risk for lung nodules.
In our study, 36.5% of participants had at least one non-calcified lung nodule. This prevalence was between the result of the Dutch-Belgian lung cancer screening trial (Nelson), where 51% of participants had non-calcified nodules [25] and 27.3% reported by the National lung screening study (NLST) [26]. The main explanations for this difference are the differences in nodule detection criteria between the studies, with detection thresholds of 15 mm3 (Nelson), 30 mm3 (current study), and 4 mm (roughly 34 mm3, NLST). However, we would expect a lower prevalence of lung nodules in our study, as in our study younger participants without a limit of pack-years were included, while the Nelson and NLST studies included participants, aged 50 to 74 years, who had a history of at least 15 or 30 pack-years of smoking [26, 27].
In total, 58% of participants had emphysema, which is almost similar to 61% as reported in the COPDGene Study using the American population [28]. This is remarkable, since the participants in those studies had a longer cumulative tobacco exposure of 42 pack-years in current and former smokers, while in our population, participants had a mean of 8 pack-years and 67% of them were never-smokers. It is worth noting that 65% of participants with emphysema only had trace emphysema. The much more severe outdoor air pollution and indoor cooking fume in China (Beijing-Tianjin-Hebei region) compared to western countries [29, 30] may play an important role in emphysema formation since there is evidence showing outdoor air pollution and indoor cooking fume contribute to higher incidence and prevalence of emphysema [31].
We found that participants with emphysema had more than one-and-a-half-fold risk for the presence of lung nodules compared to participants without emphysema, which is somewhat higher than a previous study in which a non-significant risk was reported (OR: 1.18, 95% CI: 0.74–1.73) [14]. In our study, participants with PSE seemed to have a higher risk of lung nodules than CLE, although 95% CI overlapped. Compared with CLE, PSE is more frequently associated with marked thickening walls of bronchi with distinct airway inflammatory [22], which facilitates lung nodule formation [32]. We also observed that greater severity of CLE conferred a greater risk of lung nodules. It could be explained by the effects of smoking intensity, varied air pollution exposure, and advancing age. In the current study, regarding the malignant risk of lung cancer, participants with emphysema in our study had a 70% increased risk for positive Lung-RADS category (3 or 4). This is consistent with findings from Burnett-Hartman et al that COPD is positively associated with Lung-RADS 4 (OR, 1.78; 95% CI, 1.45–2.20) [33]. More specifically, the association found in our study was only held for participants with CLE, not PSE. Similarly, a study by Gonzalez et al showed that CLE is associated with increased lung cancer risk [34]. When we focused on the participants with lung nodules, the presence of emphysema was related to larger nodules and participants with 2–3 nodules. This is consistent with findings of a study by Ewa et al, who found that emphysema is more frequently associated with larger and multiple lung nodules [11].
Several mechanisms can explain the association between emphysema and lung nodules. Most of the nodules are not malignant in general [35]. First, local accumulations of inflammatory cells such as lymphocytes, macrophages, and neutrophils occur on external stimulus, which could facilitate lung injury resulting in emphysema and lung nodule formation [36]. Second, as the shared risk factor for emphysema and lung nodule, smoking exposure could induce oxidative stress which will cause reactive oxygen production and antioxidant reduction. The progress of oxidative stress enhances inflammation, DNA damage, and accelerated aging, which can result in emphysema, lung nodules, and ultimately lung cancer [37]. Third, air pollutants, such as nitrogen oxides and particulate matter 2.5 (PM2.5), are highly reactive oxidants and could cause long-term inflammation [38]. In addition, PM2.5 could also induce changes in long-noncoding RNAs through reactive oxygen species, thereby promoting autophagy and proliferation of lung cells, further leading to the occurrence of emphysema and lung nodules [39]. Finally, the main pathological feature of emphysema is the permanent enlargement of airspaces distal to the terminal bronchioles, which will be the risk factor for neoplastic transformation [11].
Strengths and limitations
This study is, to the best of our knowledge, the first to analyze the association between the subtypes and severity of emphysema and the presence of lung nodules based on LDCT chest CT in a general Chinese population. Moreover, the participants in this study came from a general population with no inclusion criteria regarding the smoking status and pack-years, making the results innovative and more generalizable compared to hospital-based and other population-based screening studies. Additionally, our study had a good interobserver agreement of emphysema, which is comparable to that of study using the Fleischner Society classification system (k = 0.82, 95%: 0.80–0.84) [28]. This study also has some limitations. First, the number of participants with PSE and confluent-ADE of CLE was relatively small, so we merged it with moderate severity and did not perform an analysis for PSE severity. Second, this was a cross-sectional study, which limits the ability to explore the etiological relationship between emphysema and lung nodules. Third, lung function was not measured in the current study, while this might be an important confounding factor that could compromise the OR of emphysema for lung nodules after adjusting. Fourth, there is a lack of complete follow-up for lung cancer diagnosis of all participants. To overcome this limitation, the Lung-RADS risk for malignancy of nodules was added.
Conclusion
This study shows that the presence, subtypes, and severity of emphysema are related to the presence of lung nodules in a general Chinese population. The risk for the presence of lung nodules increases with CLE severity. The presence of emphysema and specifically CLE subtype are related to the positive Lung-RADS category. The significance of these findings for lung cancer screening should be evaluated.
Abbreviations
- aOR:
-
Adjusted odds ratios
- BMI:
-
Body mass index
- CLE:
-
Centrilobular emphysema
- cOR:
-
Crude odds ratios
- LDCT:
-
Low-dose computed tomography
- Moderate-ADE:
-
Moderate, confluent or advanced destructive
- PSE:
-
Paraseptal emphysema
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Zheng R, Zeng H, Zuo T et al (2016) Lung cancer incidence and mortality in China, 2011. Thorac Cancer 7:94–99
Cao M, Chen W (2019) Epidemiology of lung cancer in China. Thorac Cancer 10:3–7
de Koning HJ, van der Aalst CM, de Jong PA et al (2020) Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382:503–513
Rampinelli C, De Marco P, Origgi D et al (2017) Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 356:j347
Pedersen JH, Ashraf H, Dirksen A et al (2009) The Danish randomized lung cancer CT screening trial—overall design and results of the prevalence round. J Thorac Oncol 4:608–614
Walter JE, Heuvelmans MA, Oudkerk M (2017) Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening. Transl Lung Cancer Res 6:42–51
Liu Y, Luo H, Qing H et al (2019) Screening baseline characteristics of early lung cancer on low-dose computed tomography with computer-aided detection in a Chinese population. Cancer Epidemiol 62:101567
Wilson DO, Weissfeld JL, Fuhrman CR et al (2008) The Pittsburgh Lung Screening Study (PLuSS) outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 178:956–961
Albert RH, Russell JJ (2009) Evaluation of the solitary pulmonary nodule. Am Fam Physician 80:827–831
Wachuła E, Szabłowska-Siwik S, Czyżewski D, Kozielski J, Adamek M (2020) Emphysema affects the number and characteristics of solitary pulmonary nodules identified by chest low-dose computed tomography. A study on screenees with high-risk lung cancer recruited in Upper Silesia. Polskie Arch Med 130:17–24
de Torres JP, Bastarrika G, Wisnivesky JP et al (2007) Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 132:1932–1938
Koshiol J, Rotunno M, Consonni D et al (2009) Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One 4:e7380
Hohberger LA, Schroeder DR, Bartholmai BJ et al (2014) Correlation of regional emphysema and lung cancer: a lung tissue research consortium-based study. J Thorac Oncol 9:639–645
Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ (2007) Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 176:285–290
Du Y, Zhao Y, Sidorenkov G et al (2019) Methods of computed tomography screening and management of lung cancer in Tianjin: design of a population-based cohort study. Cancer Biol Med 16:181–188
Oudkerk M, Devaraj A, Vliegenthart R et al (2017) European position statement on lung cancer screening. Lancet Oncol 18:e754–e766
Du Y, Li Q, Sidorenkov G et al (2021) Computed tomography screening for early lung cancer, COPD and cardiovascular disease in Shanghai: rationale and design of a population-based comparative study. Acad Radiol 28:36–45
Kastner J, Hossain R, Jeudy J et al (2021) Lung-RADS Version 1.0 versus Lung-RADS Version 1.1: comparison of categories using nodules from the National Lung Screening Trial. Radiology 300:199–206
Martini K, Frauenfelder T (2020) Advances in imaging for lung emphysema. Ann Transl Med 8:1467
Lynch DA, Moore CM, Wilson C et al (2018) CT-based visual classification of emphysema: association with mortality in the COPDGene study. Radiology 288:859–866
Lynch DA, Austin JH, Hogg JC et al (2015) CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology 277:192–205
Tammemagi MC, Katki HA, Hocking WG et al (2013) Selection criteria for lung-cancer screening. N Engl J Med 368:728–736
Liang H, Guan P, Yin Z, Li X, He Q, Zhou B (2009) Risk of lung cancer following nonmalignant respiratory conditions among nonsmoking women living in Shenyang, Northeast China. J Womens Health (Larchmt) 18:1989–1995
van Klaveren RJ, Oudkerk M, Prokop M et al (2009) Management of lung nodules detected by volume CT scanning. N Engl J Med 361:2221–2229
Team NLSTR (2013) Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 368:1980–1991
Horeweg N, Van Der Aalst CM, Thunnissen E et al (2013) Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 187:848–854
El Kaddouri B, Strand MJ, Baraghoshi D et al (2020) Fleischner Society visual emphysema CT patterns help predict progression of emphysema in current and former smokers: results from the COPDGene Study. Radiology:441–449
Zhang J, Smith KR (2003) Indoor air pollution: a global health concern. Br Med Bull 68:209–225
Zhang JJ, Samet JM (2015) Chinese haze versus Western smog: lessons learned. J Thorac Dis 7:3–13
Liu Y, Lee K, Perez-Padilla R, Hudson N, Mannino D (2008) Outdoor and indoor air pollution and COPD-related diseases in high-and low-income countries. Int J Tuberc Lung Dis 12:115–127
Houghton AM, Quintero PA, Perkins DL et al (2006) Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 116:753–759
Burnett-Hartman AN, Carroll NM, Honda SA et al (2022) Community-based lung cancer screening results in relation to patient and radiologist characteristics: the PROSPR Consortium. Ann Am Thorac Soc 19:433–441
Gonzalez J, Henschke CI, Yankelevitz DF et al (2019) Emphysema phenotypes and lung cancer risk. PLoS One 14:e0219187
Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA (2013) What do you mean, a spot?: A qualitative analysis of patients' reactions to discussions with their physicians about pulmonary nodules. Chest 143:672–677
Sze MA, Hogg JC (2015) Reply: The lung immune response to bacteria in chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 192:903–904
Brody JS, Spira A (2006) State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc Am Thorac Soc 3:535–537
Brauer M, Avila-Casado C, Fortoul TI, Vedal S, Stevens B, Churg A (2001) Air pollution and retained particles in the lung. Environ Health Perspect 109:1039–1043
Deng X, Feng N, Zheng M et al (2017) PM2. 5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim Biophys Acta 1861:112–125
Acknowledgements
Thanks to Tianjin Medical University Cancer Institute and Hospital for providing data of all participants in this study. Thanks to Yihui Du, PhD, from University Medical Center Groningen for suggestions of manuscript revision and statistics support.
Funding
This study has received funding by the Royal Netherlands Academy of Arts and Sciences (Grant No. PSA_SA_BD_01) and the Ministry of Science and Technology of the People’s Republic of China, National Key R & D Program of China (Grant No.2016YFE0103000). Xiaofei Yang was funded by Chinse Scholarship Council (CSC No.: 201807660006).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Geertruida H. de Bock.
Conflict of interest
Dr. Vliegenthart reports grants from Siemens Healthineers and all outside the submitted work;
Dr. Groen reports grants from Novartis, Eli Lilly, Siemens, and BMS and all outside the submitted work.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Only if the study is on human subjects:
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• cross sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 18.2 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, X., Dorrius, M.D., Jiang, W. et al. Association between visual emphysema and lung nodules on low-dose CT scan in a Chinese Lung Cancer Screening Program (Nelcin-B3). Eur Radiol 32, 8162–8170 (2022). https://doi.org/10.1007/s00330-022-08884-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08884-3