Abstract
Objectives
Develop and evaluate the performance of deep learning and linear regression cascade algorithms for automated assessment of the image layout and position of chest radiographs.
Methods
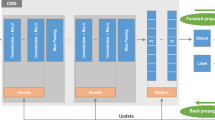
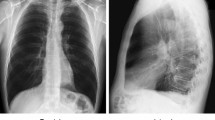
This retrospective study used 10 quantitative indices to capture subjective perceptions of radiologists regarding image layout and position of chest radiographs, including the chest edges, field of view (FOV), clavicles, rotation, scapulae, and symmetry. An automated assessment system was developed using a training dataset consisting of 1025 adult posterior-anterior chest radiographs. The evaluation steps included: (i) use of a CNN framework based on ResNet - 34 to obtain measurement parameters for quantitative indices and (ii) analysis of quantitative indices using a multiple linear regression model to obtain predicted scores for the layout and position of chest radiograph. In the testing dataset (n = 100), the performance of the automated system was evaluated using the intraclass correlation coefficient (ICC), Pearson correlation coefficient (r), mean absolute difference (MAD), and mean absolute percentage error (MAPE).
Results
The stepwise regression showed a statistically significant relationship between the 10 quantitative indices and subjective scores (p < 0.05). The deep learning model showed high accuracy in predicting the quantitative indices (ICC = 0.82 to 0.99, r = 0.69 to 0.99, MAD = 0.01 to 2.75). The automatic system provided assessments similar to the mean opinion scores of radiologists regarding image layout (MAPE = 3.05%) and position (MAPE = 5.72%).
Conclusions
Ten quantitative indices correlated well with the subjective perceptions of radiologists regarding the image layout and position of chest radiographs. The automated system provided high performance in measuring quantitative indices and assessing image quality.
Key Points
• Objective and reliable assessment for image quality of chest radiographs is important for improving image quality and diagnostic accuracy.
• Deep learning can be used for automated measurements of quantitative indices from chest radiographs.
• Linear regression can be used for interpretation-based quality assessment of chest radiographs.






Similar content being viewed by others
Abbreviations
- CNN:
-
Convolutional neural network
- DL:
-
Deep learning
- ICC:
-
Intraclass correlation coefficient
- MAD:
-
Mean absolute difference
- MAPE:
-
Mean absolute percentage error
- MOS:
-
Mean opinion score
- PCK:
-
Percentage of correct key points
References
Mettler FA Jr, Mahesh M, Bhargavan-Chatfield M et al (2020) Patient exposure from radiologic and nuclear medicine procedures in the United States: Procedure volume and effective dose for the period 2006-2016. Radiology 295:418–427
Tesselaar E, Dahlström N, Sandborg M (2016) Clinical audit of image quality in radiology using visual grading characteristics analysis. Radiat Prot Dosimetry 169:340–346
Whaley JS, Pressman BD, Wilson JR, Bravo L, Sehnert WJ, Foos DH (2013) Investigation of the variability in the assessment of digital chest X-ray image quality. J Digit Imaging 26:217–226
Andersen ER, Jorde J, Taoussi N, Yaqoob SH, Konst B, Seierstad T (2012) Reject analysis in direct digital radiography. Acta Radiol 53:174–178
Miyata T, Yanagawa M, Hata A, Honda O, Tomiyama N (2020) Influence of field of view size on image quality: ultra-high-resolution CT vs. conventional high-resolution CT. European Radiology 30:3324–3333
Hardy M, Scotland B, Herron L (2015) Assessing sagittal rotation on posteroanterior chest radiographs: the effect of body morphology on radiographic appearances. J Med Imaging Radiat Sci 46:365–371
Vañó E, Guibelalde E, Morillo A, Alvarez-Pedrosa CS, Fernández JM (1995) Evaluation of the European image quality criteria for chest examinations. Br J Radiol 68:1349–1355
Cui S, Ming S, Lin Y et al (2020) Development and clinical application of deep learning model for lung nodules screening on CT images. Sci Rep 10:13657
Ye Q, Shen Q, Yang W et al (2020) Development of automatic measurement for patellar height based on deep learning and knee radiographs. Eur Radiol 30:4974–4984
Xue J, Wang B, Ming Y et al (2020) Deep learning-based detection and segmentation-assisted management of brain metastases. Neuro Oncol 22:505–514
Higaki T, Nakamura Y, Tatsugami F, Nakaura T, Awai K (2019) Improvement of image quality at CT and MRI using deep learning. Jpn J Radiol 37:73–80
Satyananda Kashyap MM, Karargyris A, Wu JT, Morris M, Babak Saboury ES, Syeda-Mahmood T (2019) Artificial intelligence for point of care radiograph quality assessment. Conference: SPIE Medical Imaging 2019 At: San Diego
Nousiainen K, Mäkelä T, Piilonen A, Peltonen JI (2021) Automating chest radiograph imaging quality control. Phys Med 83:138–145
Poggenborg J, Yaroshenko A, Wieberneit N, Harder T, Gossmann A (2021) Impact of AI-based real time image quality feedback for chest radiographs in the clinical routine. medRxiv. https://doi.org/10.1101/2021.06.10.21258326 2021.2006.2010.21258326
Commission of the European Communities (1996) European guidelines on quality criteria for diagnostic radiographic images, EUR 16260, Brussels
Ganten M, Radeleff B, Kampschulte A, Daniels MD, Kauffmann GW, Hansmann J (2003) Comparing image quality of flat-panel chest radiography with storage phosphor radiography and film-screen radiography. AJR Am J Roentgenol 181:171–176
Ronneberger O, Fischer P, Brox T (2015) U-Net: convolutional networks for biomedical image segmentation. Springer International Publishing, Cham, pp 234–241
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), 2016, pp 770–778
Nair V, Hinton GE (2010) Rectified linear units improve restricted boltzmann machines vinod nairproceedings of the 27th International Conference on Machine Learning (ICML-10), June 21-24, 2010, Haifa, Israel
Ioffe S, Szegedy C (2015) Batch normalization: accelerating deep network training by reducing internal covariate shift. http://arxiv.org/abs/1502.03167
Russakovsky O, Deng J, Su H et al (2014) ImageNet large scale visual recognition challenge. In: arXiv e-prints. Available via http://arxiv.org/abs/1409.0575. Accessed 13 Jul 2021
Abadi M, Barham P, Chen J et al (2016) Tensorflow: a system for large-scale machine learning12th {USENIX} symposium on operating systems design and implementation ({OSDI} 16), pp 265-283
Kingma DP, Ba J (2014) Adam: a method for stochastic optimization. In: arXiv e-prints. Available via https://arxiv.org/abs/1412.6980. Accessed 13 Jul 2021
Chen Y, Wang Z, Peng Y, Zhang Z, Yu G, Sun J (2017) Cascaded pyramid network for multi-person pose estimation. In: arXiv e-prints. Available via https://arxiv.org/abs/1711.07319. Accessed 13 Jul 2021
Payer C, Štern D, Bischof H, Urschler M (2019) Integrating spatial configuration into heatmap regression based CNNs for landmark localization. Med Image Anal 54:207–219
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Amdisen A (1987) Pearson’s correlation coefficient, p-value, and lithium therapy. Biol Psychiatry 22:926–928
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Larson DB, Chen MC, Lungren MP, Halabi SS, Stence NV, Langlotz CP (2018) Performance of a deep-learning neural network model in assessing skeletal maturity on pediatric hand radiographs. Radiology 287:313–322
Krupinski EA (2010) Current perspectives in medical image perception. Atten Percept Psychophys 72:1205–1217
Båth M, Sund P, Månsson LG (2002) Evaluation of the imaging properties of two generations of a CCD-based system for digital chest radiography. Med Phys 29:2286–2297
Bier B, Goldmann F, Zaech JN et al (2019) Learning to detect anatomical landmarks of the pelvis in X-rays from arbitrary views. Int J Comput Assist Radiol Surg 14:1463–1473
Berg JV, Krnke S, Gooen A, Bystrov DB, Young S (2020) Robust chest x-ray quality assessment using convolutional neural networks and atlas regularization. Proc. SPIE 11313, Medical Imaging 2020: Image Processing, 113131L (10 March 2020). https://doi.org/10.1117/12.2549541
Hou W, Gao X, Tao D, Li X (2015) Blind image quality assessment via deep learning. IEEE Trans Neural Netw Learn Syst 26:1275–1286
Stępień I, Obuchowicz R, Piórkowski A, Oszust M (2021) Fusion of deep convolutional neural networks for no-reference magnetic resonance image quality assessment. Sensors (Basel) 21:1043
Acknowledgements
We would like to thank all the involved professional image quality assessment practitioners (radiologists and technicians) for dedicating their time and skill to the completion of this study.
Funding
This study was funded by Key Research and Development Project of Zhejiang Province of China (2020C01058).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Xiangyang Gong.
Conflict of interest
Hongli Ji, Linyang He, and Guohua Cheng are employees of Hangzhou Jianpei Technology Company Ltd. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• experimental study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 13549 kb)
Rights and permissions
About this article
Cite this article
Meng, Y., Ruan, J., Yang, B. et al. Automated quality assessment of chest radiographs based on deep learning and linear regression cascade algorithms. Eur Radiol 32, 7680–7690 (2022). https://doi.org/10.1007/s00330-022-08771-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08771-x