Abstract
Objectives
To assess diagnostic accuracy of automated 3D volumetry of cardiac chambers based on computed tomography pulmonary angiography (CTPA) for the differentiation of pulmonary hypertension due to left heart disease (group 2 PH) from non-group 2 PH compared to manual diameter measurements.
Methods
Patients with confirmed PH undergoing right heart catheterisation and CTPA within 100 days for diagnostic workup of PH between August 2013 and February 2016 were included in this retrospective, single-centre study. Automated 3D segmentation of left atrium, left ventricle, right atrium and right ventricle (LA/LV/RA/RV) was performed by two independent and blinded radiologists using commercial software. For comparison, axial diameters were manually measured. The ability to differentiate group 2 PH from non-group 2 PH was assessed by means of logistic regression.
Results
Ninety-one patients (median 67.5 years, 44 women) were included, thereof 19 patients (20.9%) classified as group 2 PH. After adjustment for age, sex and mean pulmonary arterial pressure, group 2 PH was significantly associated with larger LA volume (p < 0.001), larger LV volume (p = 0.001), lower RV/LV volume ratio (p = 0.04) and lower RV/LA volume ratio (p = 0.003). LA volume demonstrated the highest discriminatory ability to identify group 2 PH (AUC, 0.908; 95% confidence interval, 0.835–0.981) and was significantly superior to LA diameter (p = 0.009). Intraobserver and interobserver agreements were excellent for all volume measurements (intraclass correlation coefficients 0.926–0.999, all p < 0.001).
Conclusions
LA volume quantified by automated, CTPA-based 3D volumetry can differentiate group 2 PH from other PH groups with good diagnostic accuracy and yields significantly higher diagnostic accuracy than left atrial diameter.
Key Points
• Automated cardiac chamber volumetry using non-gated CT pulmonary angiography can differentiate pulmonary hypertension due to left heart disease from other causes with good diagnostic accuracy.
• Left atrial volume yields significantly higher diagnostic accuracy than left atrial axial diameter for identification of pulmonary hypertension due to left heart disease without time-consuming manual processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary hypertension (PH) is a hemodynamic condition defined as a pathological increase of the mean pulmonary arterial pressure (mPAP) at rest measured by right heart catheterisation (RHC) [1]. The World Health Organization (WHO) classification of PH differentiates five groups based on similarities in aetiology, hemodynamic profile, clinical findings and treatment strategy [2]. Hemodynamically, PH can be subdivided into pre-capillary, post-capillary or combined pre- and post-capillary PH [3, 4].
PH due to left heart disease (WHO group 2 PH), a form of post-capillary PH, is a frequent complication of left heart disease (LHD) and associated with worse prognosis [5, 6]. Management of group 2 PH differs from that of other PH groups and should focus on treatment of the underlying cardiac disorder [3]. The diagnosis of group 2 PH is primarily defined by an elevated pulmonary arterial wedge pressure (PAWP) > 15 mmHg at rest measured by RHC [1]. However, guidelines recommend that estimation of clinical pre-test probability for LHD based on non-invasive parameters should precede invasive catheterisation for improved differentiation of group 2 PH from other causes and improved indication of invasive right and left heart catheterisation [3].
Computed tomography (CT), especially CT pulmonary angiography (CTPA), is frequently acquired in patients with suspected PH to rule out pulmonary embolism and parenchymal lung disease. Previous studies suggested good diagnostic accuracy of enlarged left atrial diameter and area measured on CTPA for identification of group 2 PH [7,8,9]. However, volumetry of cardiac chambers might be more accurate and reliable in detecting cardiac chamber enlargement [10]. Technical advances have enabled automated volumetry of cardiac chambers based on CTPA [11,12,13], alleviating the need for time-consuming manual processing. Only a limited number of studies investigated the ability of cardiac chamber volumes to differentiate group 2 PH from other causes [7, 14, 15] without comparison to manual diameter measurements within the same patient cohort, to our knowledge.
The purpose of this study was to assess the diagnostic accuracy of automated 3D volumetry of the cardiac chambers based on CTPA for the differentiation of WHO group 2 PH from other PH groups (non-group 2 PH). Furthermore, we aimed to test our hypothesis that automated 3D volumetry yields higher diagnostic accuracy than manual diameter measurements of cardiac chambers.
Materials and methods
Patients
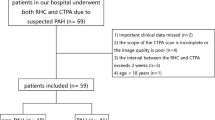
The retrospective, single-centre study was approved by the institutional review board, and patient informed consent was waived. Records of all patients who underwent RHC and CTPA for the diagnostic workup of PH in our institution between August 2013 and February 2016 were reviewed. CTPA was performed to diagnose or rule out underlying causes, in particular chronic thromboembolic disease. Diagnosis and classification were made according to the current ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension [1]. All patients with PH confirmed by RHC were included. Exclusion criteria were an interval between CTPA and RHC of more than 100 days, non-diagnostic CTPA and failure of 3D segmentation (Fig. 1). RHC measurements (mPAP and PAWP), WHO classification and patient characteristics (sex, height, weight, age) were extracted from the records. Body surface area (BSA) was calculated according to the formula by Du Bois and Du Bois [16].
Right heart catheterisation
RHC was performed according to guidelines [1] by two interventional pulmonologists each with more than 7 years of experience in RHC. A 7-French pulmonary artery catheter was introduced via an 8-French introducer sheath in the right internal jugular vein. Pulmonary arterial pressure measurements were recorded after zero levelling according to the guidelines [1].
CTPA acquisition
Non-gated CTPA data were acquired using a 64-detector CT scanner (Somatom Definition AS 64, Siemens Healthineers) in inspiratory breath-hold in supine position. Protocol settings were as follows: automated tube voltage selection (80–140 kVp), automated tube current modulation (reference tube current of 100 mAs at 120 kVp), bolus tracking in the main pulmonary artery, 50 ml of iodinated contrast agent (Ultravist 300, Bayer HealthCare) followed by a saline bolus of 50 ml with identical injection rate of 3–5 ml/s depending on venous access, collimation of 64 × 0.6 mm, iterative reconstruction kernel I40f/3 and reconstructed slice thickness of 1 mm with 0.7-mm increments. Scan length and field of view were adjusted to include the whole chest of each patient.
Image analysis
Automated segmentation and volumetry of cardiac chambers was performed using a commercially available, model-based algorithm previously validated on gated CT scans [13, 17] (CT Pulmonary Artery Analysis, Intellispace Portal V11, Philips Healthcare). The algorithm automatically computes 3D segmentations of left and right atria (LA, RA) and ventricles (LV, RV). Pulmonary veins and the left atrial appendage were automatically excluded from the LA segmentation. Two board-certified radiologists with 6 and 8 years of experience in cardiovascular imaging reviewed segmentations in an individual random order and blinded to any clinical data, and manually corrected visible deviations from the chambers’ contours using the brush tools provided by the same software. The average of the measurements by the two radiologists was used for further analysis. To assess intraobserver agreement, one of the two radiologists repeated the image analysis after 12 months in half of the patients.
Diameter measurements of all cardiac chambers on axial slices of the CTPA data were performed by one of the board-certified radiologists in a different random order, again blinded to any other measurements and clinical data. LV and RV diameters were measured perpendicular to the interventricular septum on the slice on which the respective ventricle appeared largest [18]. LA diameter was determined as the largest anterior-posterior-diameter on the slice in the middle 50% of the craniocaudal extension of the left atrium [19]. RA diameter was measured as the largest diameter parallel to the tricuspid valve plane, excluding right atrial appendage and coronary sinus [20]. Additionally, contrast attenuation in Hounsfield units (HU) of the LA was assessed by placing a 300 mm2 circular region of interest in the centre of the LA on the same slice as the diameter measurement.
Statistical analysis
Normal distribution was tested using the Shapiro-Wilk test and QQ-plots. Normally distributed continuous variables are described by mean ± standard deviation and 95% confidence intervals (CI), non-normally distributed continuous variables by median and interquartile range (IQR). Categorical variables are given as numbers and percentages. Interobserver and intraobserver agreements were assessed by calculation of intraclass correlation coefficients using a two-way mixed model testing for absolute agreement. Correlation between LA contrast attenuation and the segmentation error was analysed using Pearson’s correlation.
Comparisons between group 2 PH and non-group 2 PH patients were performed using the chi-square test for categorical variables, the two-sided t-test for normally distributed continuous variables and the Mann-Whitney U test for non-normally distributed continuous variables. ROC analyses were performed to assess the ability to differentiate group 2 PH from non-group 2 PH, and AUCs were calculated. Statistical differences of AUCs between volumetric and axial measurements were assessed using the two-sided test by DeLong et al for paired data [21]. All analyses were also performed for BSA-corrected volume and diameter measurements.
Linear correlation with the logit of each cardiac chamber measurement was confirmed and binary multiple logistic regression analysis was conducted for each cardiac chamber measurement separately, including age, sex and mPAP as potentially confounding covariates. After exclusion of significant collinearity between covariates, a binary logistic regression analysis including age and cardiac chamber volumes was performed using stepwise inclusion according to the likelihood ratio. AUC of the resulting model for the identification of group 2 PH was calculated. Sensitivities, specificities and positive and negative predictive values based on the prevalence of group 2 PH in our study cohort were calculated for the threshold values yielding the maximum Youden index for each measurement.
Using the propensity score method, pairwise matching of patients with group 2 PH to non-group 2 PH patients based on age, sex and mPAP using the nearest neighbour method was performed and group analysis repeated as described above.
A p value < 0.05 was considered statistically significant. All statistical analyses were accomplished using SPSS version 27.0 (SPSS Inc.) and R version 4.0.2 (R Foundation for Statistical Computing) including packages MatchIt version 4.1.0 [22] and pROC version 1.16.2 [23].
Sample size-estimation was based on Power Analysis and Sample Size (PASS 11, NCSS, LLC) [24] using the following prerequisites and assumptions: alpha level 0.05, power 0.8, prevalence of group 2 PH among the study population 20%, null hypothesis specificity 70%, alternative hypothesis specificity 90%. The calculated sample size was 39 patients including a minimum of 8 patients with group 2 PH.
Results
Study cohort
The patient characteristics of the final study population of 91 patients are summarised in Table 1. Nineteen patients (20.9%) were classified as group 2 PH, of which 18 (94.7%) were diagnosed with heart failure with preserved ejection fraction and 1 (5.3%) with heart failure with reduced ejection fraction. Seventy-two patients (79.1%) were identified having non-group 2 PH, of which 37 patients (51.4%) were classified as group 1 PH, 15 patients (20.8%) as group 3 PH and 20 patients (27.8%) as group 4 PH.
Cardiac chamber volumes and diameters
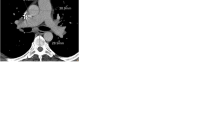
Volumes and diameters of LA, LV and RA were significantly larger in patients with group 2 PH compared to non-group 2 PH, while RV/LV, RA/LA and RV/LA volume and diameter ratios were significantly lower (Table 1, Figs. 2, 3 and 4).
Upper row (A, B) shows four-chamber view, lower row (C, D) 3D visualisations of representative cardiac chamber segmentations in a 79-year-old female patient with group 2 pulmonary hypertension (A, C) compared to a 63-year-old female patient with group 3 pulmonary hypertension (B, D). A particular enlargement of the LA is visible in the patient with group 2 pulmonary hypertension. LA, left atrium; LV, left ventricle; mPAP, mean pulmonary arterial pressure; PH, pulmonary hypertension; RA, right atrium; RV, right ventricle
Interobserver and intraobserver agreements of cardiac chamber volumetry were excellent for all cardiac chambers, with intraclass correlation coefficients of 0.951/0.926 for LV volume (95% CI 0.923–0.968/0.872–0.958, both p < 0.001), 0.978/0.980 for RV volume (95% CI 0.967–0.986/0.954–0.990, both p < 0.001), 0.969/0.983 for LA volume (95% CI 0.954–0.980/0.970–0.990, both p < 0.001) and 0.999/0.998 for RA volume (95% CI 0.998–0.999/0.997–0.999, both p < 0.001). Manual adjustments of automatic segmentations were performed in 38 of 91 patients (41.8%)/96 of 364 cardiac chambers (26.4%) by reader 1 and 45 patients (49.5%)/102 cardiac chambers (28.0%) by reader 2, yielding fully automatic segmentations of 72.8% of cardiac chambers without the need of manual adjustment. In cases of manual adjustment, average time for adjusting the segmentation of one cardiac chamber was 86.7 ± 34.2 s. The segmentation error, i.e. the difference between corrected and fully automatic volumes, was small (95% CI for LA (−3.4)–(+0.4)ml/(−6.9)–(+0.4%), for LV (−3.6)–(+2.0)ml/(−5.9)–(+1.2%), for RA (−2.7)–( −0.2)ml/(−1.3)–( −0.2%) and for RV (+7.0)–(+17.9)ml/(+2.5)–(+6.0%)).
Mean LA contrast attenuation was 288.0 HU (95% CI 267.8–308.2 HU). There was only a very weak positive, statistically non-significant correlation between LA contrast attenuation and the segmentation error for LA volume (r2 = 0.021, p = 0.17).
Identification of WHO group 2 pulmonary hypertension
ROC analysis showed excellent ability of LA volume to differentiate group 2 PH from non-group 2 PH with an AUC of 0.908 (95% CI 0.835–0.981) and good ability of LV volume, as well as RV/LV, RA/LA and RV/LA volume ratios (Table 2, Supplementary Table A). Cardiac chamber volumes demonstrated higher AUC values compared to axial diameters, with LA volume showing statistically significant superiority compared to LA diameter (p = 0.009, Table 2). Multiple logistic regression analysis demonstrated that the significance of enlarged LA volume (p < 0.001), enlarged LV volume (p = 0.001), lower RV/LV volume ratio (p = 0.04) and lower RV/LA volume ratio (p = 0.003) as predictors for group 2 PH persisted after adjustment for age, sex and mPAP (Table 3). The same was true for the respective axial diameter values (Table 3).
The stepwise logistic regression analysis for identification of group 2 PH patients resulted in the final model with LA volume (p < 0.001) and RV/LV volume ratio (p = 0.02) as covariates:
ROC analysis demonstrated an AUC of 0.936 (95% CI 0.879–0.993). The corresponding sensitivity, specificity and positive and negative predictive values using a cut-off probability of > 0.40 yielding the maximum Youden index of 1.73 were 78.9%, 94.4%, 78.9% and 94.4%, respectively (Table 4). For LA volume/LA diameter alone, sensitivity, specificity and positive and negative predictive values using a cut-off value of ≥ 110 ml/≥ 41 mm were 78.9%/89.5%, 90.3%/62.5%, 68.2%/38.6% and 94.2%/95.7%, respectively (Table 4).
The above-mentioned analyses were also conducted after adjusting cardiac chamber volumes and diameters for BSA. This did not have a relevant influence on the major findings. For example, ROC analysis to differentiate group 2 PH from non-group 2 PH yielded AUC values of 0.893 for LA volumeBSA (95% CI 0.819–0.967), 0.761 for LV volumeBSA (95% CI 0.636–0.886), 0.636 for RA volumeBSA (95% CI 0.491–0.781) and 0.447 for RV volumeBSA (95% CI 0.280–0.613).
Finally, to account for differences in clinical characteristics between the two groups, pairwise matching of group 2 (n = 19) and non-group 2 PH patients (n = 19) was performed using the propensity score method (Table 5). Analyses after pairwise matching confirmed that volumes and diameters of LA, LV and RA were significantly larger in group 2 PH patients compared to matched non-group 2 PH patients, while RV/LA volume and diameter ratios were significantly lower (Table 5).
Discussion
This study assessed the diagnostic accuracy of automated 3D volumetry of the cardiac chambers based on CTPA for the differentiation of WHO group 2 PH from other PH groups and demonstrated excellent ability of LA volume to differentiate group 2 PH from non-group 2 PH with an AUC of 0.908 and corresponding sensitivity, specificity and positive and negative predictive values at the maximum Youden index of 78.9%, 90.3%, 68.2% and 94.2%, respectively. LA volume was a significantly better predictor of group 2 PH compared to LA diameter as measured on axial CTPA slices. Diagnostic accuracy could be slightly improved by combining LA volume and RV/LV volume ratio resulting in an AUC of 0.936.
Correct diagnosis of group 2 PH can be challenging, especially in cases of heart failure with preserved ejection fraction, and the diagnostic pathway should be based on the pre-test probability of LHD [3]. As CTPA is frequently acquired in addition to echocardiography during diagnostic workup [3, 25, 26], including CTPA-based parameters into the estimation of pre-test probability of LHD appears to be a promising approach for translation into clinical practice and may improve confidence when deciding which patient may require invasive catheterisation. LA area and volume can also be estimated by transthoracic echocardiography using planar measurements and geometric assumptions [3, 25]. However, LA volume estimation by echocardiography has been shown to be less accurate compared to CT-based volume measurements [26].
One previous study applied the software for cardiac chamber volume analysis also used in our study in a similar clinical setting and patient cohort size [14]. This previous study found a similar AUC value of 0.92 using LA volume for identification of group 2 PH, but did not perform diameter measurements for comparison [14]. Our study not only provides confirmatory evidence for the diagnostic accuracy of LA volume in a different institution and patient cohort but also demonstrates its superiority compared to LA axial anteroposterior diameter in an intra-individual comparison. LA anteroposterior diameter is the most widely used LA measurement in clinical practice and PH research in both echocardiography and CT according to international recommendations and literature [7, 19, 27,28,29,30]. Previous studies reported AUC values of 0.71–0.83 for identification of group 2 PH by LA anteroposterior diameter, compared to 0.83 in our study cohort [7, 8].
LA transverse diameter represents an alternative but has only been investigated in a single study regarding identification of group 2 PH [8]. LA area measurements have also been proposed to differentiate group 2 PH from non-group 2 PH with reported AUC values of 0.73–0.85 being slightly higher compared to LA anteroposterior diameter, but required time-consuming manual delineation of the LA thus hampering its clinical use [7,8,9]. In our study, the software provided robust segmentations without the need of manual adjustment in 72.8% of cardiac chambers and tools for manual adjustments with little time and effort. Fully automatic segmentation without any manual adjustment resulted in only marginally lower diagnostic accuracy with an AUC of 0.90 for LA volume.
The advantage of volume measurements over one- or two-dimensional measurements has also been demonstrated for other body regions [31,32,33]. This may be attributable to the lower susceptibility to geometric variances in asymmetrical geometries, as has also been discussed for the left atrium [10]. Prospective studies are needed to confirm our findings.
This study is subject to some limitations. A retrospective study might be prone to confounders and selection bias. However, we aimed to control for potential confounders by including respective covariates in multiple regression analysis and for potential group differences in the baseline characteristics by performing propensity score matching. Diagnosis and classification of PH groups in this study were based on the current ESC/ERS guidelines [1]. While the 6th World Symposium on Pulmonary Hypertension suggested some changes to the PH definitions, these suggestions have not yet been integrated into the ESC/ERS guidelines [4]. The findings of our study might differ to some degree should the new recommendations be adopted by the guidelines in the future. Furthermore, non-gated CTPA was used for cardiac chamber segmentation. The model-based algorithm was previously evaluated on gated and non-gated CT scans and found to be accurate and highly reliable [13, 14, 17]. Gated CT scans, however, are less frequently acquired in clinical routine, may have higher radiation exposure and be less beneficial for LA volume measurements given the smaller amount of LA motion during the cardiac cycle compared to LV or RV motion. Contrast enhancement of the cardiac chambers was sufficient for reproducible measurements as demonstrated by the excellent interobserver and intraobserver agreements, as well as the small segmentation error and its non-significant correlation with LA contrast attenuation. The 95% CI of LA attenuation (267.8–308.2 HU) was well above the threshold of 210 HU for MPA attenuation considered as a diagnostic CTPA scan [34]. Of note, we observed a slight tendency of the algorithm to underestimate RV volume in PH patients. Finally, in accordance with previous studies, the present study did not address patients with isolated post-capillary PH and patients with combined pre- and post-capillary PH separately [7,8,9].
In conclusion, automated 3D volumetry of cardiac chambers based on non-gated CTPA, in particular LA volume, can differentiate WHO group 2 PH patients from other PH groups with good diagnostic accuracy. LA volume yields significantly higher diagnostic accuracy than LA diameter as measured on axial CTPA slices. If proved reliable in prospective studies, cardiac chamber volumetry based on CTPA may improve non-invasive classification of PH due to LHD and thereby optimise diagnostic pathways.
Abbreviations
- CTPA:
-
CT pulmonary angiography
- LA:
-
Left atrium
- LHD:
-
Left heart disease
- LV:
-
Left ventricle
- PH:
-
Pulmonary hypertension
- RA:
-
Right atrium
- RHC:
-
Right heart catheterisation
- RV:
-
Right ventricle
References
Galiè N, Humbert M, Vachiery J-L et al (2015) 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 37:67–119
Simonneau G, Gatzoulis MA, Adatia I et al (2013) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62:D34–D41
Vachiery J-L, Tedford RJ, Rosenkranz S et al (2019) Pulmonary hypertension due to left heart disease. Eur Respir J 53:1801897
Simonneau G, Montani D, Celermajer DS et al (2019) Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 53:1801913
Rosenkranz S, Gibbs JSR, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery J-L (2016) Left ventricular heart failure and pulmonary hypertension. Eur Heart J 37:942–954
Wijeratne DT, Lajkosz K, Brogly SB et al (2018) Increasing incidence and prevalence of World Health Organization groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes 11:e003973
Currie BJ, Johns C, Chin M et al (2018) CT derived left atrial size identifies left heart disease in suspected pulmonary hypertension: derivation and validation of predictive thresholds. Int J Cardiol 260:172–177
Jivraj K, Bedayat A, Sung YK et al (2017) Left atrium maximal axial cross-sectional area is a specific computed tomographic imaging biomarker of World Health Organization group 2 pulmonary hypertension. J Thorac Imaging 32:121–126
Katikireddy CK, Singh M, Muhyieddeen K, Acharya T, Ambrose JA, Samim A (2016) Left atrial area and right ventricle dimensions in non-gated axial chest CT can differentiate pulmonary hypertension due to left heart disease from other causes. J Cardiovasc Comput Tomogr 10:246–250
Tsang TSM, Abhayaratna WP, Barnes ME et al (2006) Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 47:1018–1023
Ecabert O, Peters J, Schramm H et al (2008) Automatic model-based segmentation of the heart in CT images. IEEE Trans Med Imaging 27:1189–1201
Lorenz C, Berg JV (2006) A comprehensive shape model of the heart. Med Image Anal 10:657–670
Abadi S, Roguin A, Engel A, Lessick J (2010) Feasibility of automatic assessment of four-chamber cardiac function with MDCT: initial clinical application and validation. Eur J Radiol 74:175–181
Aviram G, Rozenbaum Z, Ziv-Baran T et al (2017) Identification of pulmonary hypertension caused by left-sided heart disease (World Health Organization group 2) based on cardiac chamber volumes derived from chest CT imaging. Chest 152:792–799
Crawley SF, Johnson MK, Dargie HJ, Peacock AJ (2013) LA volume by CMR distinguishes idiopathic from pulmonary hypertension due to HFpEF. JACC Cardiovasc Imaging 6:1120–1121
Du Bois D, Du Bois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871
Mao SS, Li D, Vembar M et al (2014) Model-based automatic segmentation algorithm accurately assesses the whole cardiac volumetric parameters in patients with cardiac CT angiography: a validation study for evaluating the accuracy of the Workstation software and establishing the reference values. Acad Radiol 21:639–647
Lu MT, Demehri S, Cai T et al (2012) Axial and reformatted four-chamber right ventricle–to–left ventricle diameter ratios on pulmonary CT angiography as predictors of death after acute pulmonary embolism. AJR Am J Roentgenol 198:1353–1360
Lick AN, Danrad R, Smith DL, Lammi MR (2017) Left atrium measurements via computed tomography pulmonary angiogram as a predictor of diastolic dysfunction. J Comput Assist Tomogr 41:792–797
Eifer DA, Nguyen ET, Thavendiranathan P, Hanneman K (2018) Diagnostic accuracy of sex-specific chest CT measurements compared with cardiac MRI findings in the assessment of cardiac chamber enlargement. AJR Am J Roentgenol 211:993–999
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Ho D, Imai K, King G, Stuart EA (2011) MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 42:1–28
Robin X, Turck N, Hainard A et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinforma 12:77–78
Bujang MA, Adnan TH (2016) Requirements for minimum sample size for sensitivity and specificity analysis. J Clin Diagn Res 10:YE01–YE06
Hussain N, Charalampopoulos A, Ramjug S et al (2016) Pulmonary hypertension in patients with heart failure and preserved ejection fraction: differential diagnosis and management. Pulm Circ 6:3–14
Arsanjani R, Flint N, Beigel R et al (2019) Comparison of accuracy of left atrial area and volume by two-dimensional trans-thoracic echocardiography versus computed tomography. Am J Cardiol 123:1180–1184
Stojanovska J, Cronin P, Patel S et al (2011) Reference normal absolute and indexed values from ECG-gated MDCT: left atrial volume, function, and diameter. AJR Am J Roentgenol 197:631–637
Taina M, Rissanen V, Hedman M et al (2015) Diagnostic performance of left atrial diameter measurement in computed tomography to detect increased left atrial volume. In Vivo 29:487–491
Mahabadi AA, Truong QA, Schlett CL et al (2010) Axial area and anteroposterior diameter as estimates of left atrial size using computed tomography of the chest: comparison with 3-dimensional volume. J Cardiovasc Comput Tomogr 4:49–54
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
Rengier F, Wörz S, Melzig C et al (2016) Automated 3D volumetry of the pulmonary arteries based on magnetic resonance angiography has potential for predicting pulmonary hypertension. PLoS One 11:e0162516
Melzig C, Wörz S, Egenlauf B et al (2019) Combined automated 3D volumetry by pulmonary CT angiography and echocardiography for detection of pulmonary hypertension. Eur Radiol 29:6059–6068
Kontopodis N, Metaxa E, Papaharilaou Y, Georgakarakos E, Tsetis D, Ioannou CV (2014) Value of volume measurements in evaluating abdominal aortic aneurysms growth rate and need for surgical treatment. Eur J Radiol 83:1051–1056
Wittram C (2007) How I do it: CT pulmonary angiography. AJR Am J Roentgenol 188:1255–1261
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the German Center for Lung Research (DZL) through grants from the German Ministry for Education and Science (BMBF; 82DZL00401, 82DZL00402, 82DZL00404).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Fabian Rengier.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
The present study forms part of an ongoing institutional investigation on imaging in patients with known or suspected PH based on an institutional database. Seventy patients (out of the 91 patients included in the present study) were included in a prior study analysing the pulmonary arteries, but not the cardiac chambers. None of the measurements reported in the present study formed part of this prior study. Reference of this prior study: Melzig, C., Wörz, S., Egenlauf, B. et al Combined automated 3D volumetry by pulmonary CT angiography and echocardiography for detection of pulmonary hypertension. Eur Radiol 29, 6059–6068 (2019). https://doi.org/10.1007/s00330-019-06188-7. The study cohort also partially overlaps with a further study based on the same institutional database, including cardiac chamber measurements of the present study, analysing a different outcome. Reference of an interim analysis of the latter study: Fink, M.A., Melzig, C., Egenlauf, B. et al Machine learning-based cardiac chamber segmentation in CTPA for the noninvasive detection of pulmonary hypertension. ECR 2020 Book of Abstracts, p. 575, Insights Imaging 11(Suppl 1), 34 (2020). https://doi.org/10.1186/s13244-020-00851-0.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 17 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Melzig, C., Do, T.D., Egenlauf, B. et al. Diagnostic accuracy of automated 3D volumetry of cardiac chambers by CT pulmonary angiography for identification of pulmonary hypertension due to left heart disease. Eur Radiol 32, 5222–5232 (2022). https://doi.org/10.1007/s00330-022-08663-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08663-0