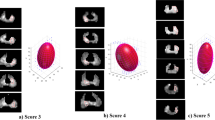
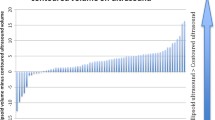
Abstract
Objective
A reliable estimation of prostate volume (PV) is essential to prostate cancer management. The objective of our multi-rater study was to compare intra- and inter-rater variability of PV from manual planimetry and ellipsoid formulas.
Methods
Forty treatment-naive patients who underwent prostate MRI were selected from a local database. PV and corresponding PSA density (PSAd) were estimated on 3D T2-weighted MRI (3 T) by 7 independent radiologists using the traditional ellipsoid formula (TEF), the newer biproximate ellipsoid formula (BPEF), and the manual planimetry method (MPM) used as ground truth. Intra- and inter-rater variability was calculated using the mixed model–based intraclass correlation coefficient (ICC).
Results
Mean volumes were 67.00 (± 36.61), 66.07 (± 35.03), and 64.77 (± 38.27) cm3 with the TEF, BPEF, and MPM methods, respectively. Both TEF and BPEF overestimated PV relative to MPM, with the former presenting significant differences (+ 1.91 cm3, IQ = [− 0.33 cm3, 5.07 cm3], p val = 0.03). Both intra- (ICC > 0.90) and inter-rater (ICC > 0.90) reproducibility were excellent. MPM had the highest inter-rater reproducibility (ICC = 0.999). Inter-rater PV variation led to discrepancies in classification according to the clinical criterion of PSAd > 0.15 ng/mL for 2 patients (5%), 7 patients (17.5%), and 9 patients (22.5%) when using MPM, TEF, and BPEF, respectively.
Conclusion
PV measurements using ellipsoid formulas and MPM are highly reproducible. MPM is a robust method for PV assessment and PSAd calculation, with the lowest variability. TEF showed a high degree of concordance with MPM but a slight overestimation of PV. Precise anatomic landmarks as defined with the BPEF led to a more accurate PV estimation, but also to a higher variability.
Key Points
• Manual planimetry used for prostate volume estimation is robust and reproducible, with the lowest variability between readers.
• Ellipsoid formulas are accurate and reproducible but with higher variability between readers.
• The traditional ellipsoid formula tends to overestimate prostate volume.






Similar content being viewed by others
Change history
23 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00330-022-08688-5
Abbreviations
- AP:
-
Antero-posterior
- BPEF:
-
Biproximate ellipsoid formula
- CsPCa:
-
Clinically significant prostate cancer
- DRE:
-
Digital rectal exam
- ICC:
-
Intraclass correlation coefficient
- IQ:
-
Interquartile
- MPM:
-
Manual planimetry measurement
- PCa:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- PSAd:
-
Prostate-specific antigen density (mpmPSAd, tefPSAd, and bpefPSAd are PSAd obtained using a volume estimated, respectively, by MPM, TEF, and BPEF methods)
- PV:
-
Prostate volume
- rSTD:
-
Relative standard deviation
- T:
-
Tesla
- T2W:
-
T2-weighted
- TEF:
-
Traditional ellipsoid formula
- TRUS:
-
Transrectal ultrasound
References
Benson MC, Whang IS, Pantuck A et al (1992) Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol 147:815–816
Seaman E, Whang M, Olsson CA, Katz A, Cooner WH, Benson MC (1993) PSA density (PSAD). Role in patient evaluation and management. Urol Clin North Am 20:653–663
Distler FA, Radtke JP, Bonekamp D et al (2017) The value of PSA density in combination with PI-RADSTM for the accuracy of prostate cancer prediction. J Urol 198:575–582
Rahmouni A, Yang A, Tempany CMC et al (1992) Accuracy of in-vivo assessment of prostatic volume by MRI and transrectal ultrasonography. J Comput Assist Tomogr 16:935–940
Lee JS, Chung BH (2007) Transrectal ultrasound versus magnetic resonance imaging in the estimation of prostate volume as compared with radical prostatectomy specimens. Urol Int 78:323–327
Paterson NR, Lavallée LT, Nguyen LN et al (2016) Prostate volume estimations using magnetic resonance imaging and transrectal ultrasound compared to radical prostatectomy specimens. Can Urol Assoc J 10:264–268
Turkbey B, Rosenkrantz AB, Haider MA et al (2019) Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 76:340–351
Garvey B, Türkbey B, Truong H, Bernardo M, Periaswamy S, Choyke PL (2014) Clinical value of prostate segmentation and volume determination on MRI in benign prostatic hyperplasia. Diagn Interv Radiol 20:229–233
Bulman JC, Toth R, Patel AD et al (2012) Automated computer-derived prostate volumes from MR imaging data: comparison with radiologist-derived MR imaging and pathologic specimen volumes. Radiology 262:144–151
Jeong CW, Park HK, Hong, Byun SS, Lee HJ, Lee SE (2008) Comparison of prostate volume measured by transrectal ultrasonography and MRI with the actual prostate volume measured after radical prostatectomy. Urol Int 81:179–185
Mahdavi SS, Chng N, Spadinger I, Morris WJ, Salcudean SE (2011) Semi-automatic segmentation for prostate interventions. Med Image Anal 15:226–237
Aldoj N, Biavati F, Michallek F, Stober S, Dewey M (2020) Automatic prostate and prostate zones segmentation of magnetic resonance images using DenseNet-like U-net. Sci Rep 10:14315
Meyer A, Rakr M, Schindele D, Blaschke S (2019) Towards patient-individual PI-Rads v2 sector map: Cnn for automatic segmentation of prostatic zones from T2-weighted MRI. In: 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019). IEEE, Venice, Italy, pp 696–700
Wasserman NF, Niendorf E, Spilseth B (2020) Measurement of prostate volume with MRI (a guide for the perplexed): biproximate method with analysis of precision and accuracy. Sci Rep 10:575
Bezinque A, Moriarity A, Farrell C, Peabody H, Noyes SL, Lane BR (2018) Determination of prostate volume: a comparison of contemporary methods. Acad Radiol 25:1582–1587
Sosna J, Rofsky NM, Gaston SM, DeWolf WC, Lenkinski RE (2003) Determinations of prostate volume at 3-Tesla using an external phased array coil: comparison to pathologic specimens. Acad Radiol 10:846–853
Ghafoor S, Becker AS, Woo S et al (2020) Comparison of PI-RADS versions 2.0 and 2.1 for MRI-based calculation of the prostate volume. Acad Radiol 28(11):1548–1556. https://doi.org/10.1016/j.acra.2020.07.027
Turkbey B, Fotin SV, Huang RJ et al (2013) Fully automated prostate segmentation on MRI: comparison with manual segmentation methods and specimen volumes. AJR Am J Roentgenol 201:W720–W729
Mazaheri Y, Goldman DA, Di Paolo PL, Akin O, Hricak H (2015) Comparison of prostate volume measured by endorectal coil MRI to prostate specimen volume and mass after radical prostatectomy. Acad Radiol 22:556–562
Vichot F, Cochet H, Bleuzé B, Toussaint N, Jaïs P, Sermesant M (2012) Cardiac interventional guidance using multimodal data processing and visualisation: medInria as an interoperability platform. Midas Journal. https://med.inria.fr/about/the-team
Lowekamp BC, Chen DT, Ibáñez L, Blezek D (2013) The design of SimpleITK. Front Neuroinform 7:45
Yaniv Z, Lowekamp BC, Johnson HJ, Beare R (2018) SimpleITK image-analysis notebooks: a collaborative environment for education and reproducible research. J Digit Imaging 31:290–303
Scrucca L, Fop M, Murphy TB, Raftery AE (2016) mclust 5: clustering, classification and density estimation using Gaussian finite mixture models. R J 8:289–317
McGraw KO, Wong SP (1996) Forming inferences about some intraclass correlation coefficients. Psychol Methods 1:30–46
Mottet N, van den Bergh RCN, Briers E et al (2021) EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 79:243–262
Rozet F, Mongiat-Artus P, Hennequin C et al (2020) French ccAFU guidelines - update 2020-2022: prostate cancer. Prog Urol 30:S136–S251
Eri LM, Thomassen H, Brennhovd B, Håheim LL (2002) Accuracy and repeatability of prostate volume measurements by transrectal ultrasound. Prostate Cancer Prostatic Dis 5:273–278
Weinreb JC, Barentsz JO, Choyke PL et al (2016) PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 69:16–40
Jonmarker S, Valdman A, Lindberg A, Hellström M, Egevad L (2006) Tissue shrinkage after fixation with formalin injection of prostatectomy specimens. Virchows Arch 449:297–301
Orczyk C, Taneja SS, Rusinek H, Rosenkrantz AB (2014) Assessment of change in prostate volume and shape following surgical resection through co-registration of in-vivo MRI and fresh specimen ex-vivo MRI. Clin Radiol 69:e398–e403
Haas M, Günzel K, Miller K, Hamm B, Cash H, Asbach P (2017) Is the ellipsoid formula the new standard for 3-Tesla MRI prostate volume calculation without endorectal coil? Urol Int 98:49–53
Acknowledgements
We thank Julien Castelneau, software engineer of Inria, for his help in the development of MedInria Software (MedInria—medical image visualization and processing software by Inria https://med.inria.fr-RRID:SCR_001462). This work has been supported by the French government, through the 3IA Côte d’Azur Investments in the Future project managed by the National Research Agency (ANR) with the reference numbers ANR-19-P3IA-0002 and ANR-17-EURE-0004. Data were extracted from the Clinical Data Warehouse of the Greater Paris University Hospitals (Assistance Publique – Hôpitaux de Paris). The authors are grateful to the members of the AP-HP WIND and URC teams, and in particular Cyrina Saussol and Aurélien Maire.
We also thank Dr. Hari Sreedhar for a thorough proofreading of this paper.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Pr Raphaële Renard-Penna.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Dr. Benjamin Granger kindly provided statistical advice for this manuscript. Also, one of the authors, Dimitri Hamzaoui, has significant statistical expertise.
Informed consent
According to French regulation, consent was waived as the MRI were acquired as part of the routine clinical care of the patients and the design of our study was retrospective.
Ethical approval
The data were made available by the data warehouse of the AP-HP, and the study was approved by the Ethical and Scientific Board of the AP-HP (IRB00011591).
Methodology
• retrospective
• observational
• performed in 2 institutions
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the name of the author Raphaële Renard-Penna was incorrectly given as Raphaële Renard Penna. The name was also tagged incorrectly in the HTML version of this article. The author's first name is Raphaële and the family name is Renard-Penna.
Supplementary Information
ESM 1
(DOCX 58 kb)
Rights and permissions
About this article
Cite this article
Hamzaoui, D., Montagne, S., Granger, B. et al. Prostate volume prediction on MRI: tools, accuracy and variability. Eur Radiol 32, 4931–4941 (2022). https://doi.org/10.1007/s00330-022-08554-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08554-4