Abstract
Objectives
To evaluate the correlation between liver enhancement on hepatobiliary phase and liver function parameters in a multicenter, multivendor study.
Methods
A total of 359 patients who underwent gadoxetic acid–enhanced MRI using a standardized protocol with various scanners within a prospective multicenter phase II trial (SORAMIC) were evaluated. The correlation between liver enhancement on hepatobiliary phase normalized to the spleen (liver-to-spleen ratio, LSR) and biochemical laboratory parameters, clinical findings related to liver functions, liver function grading systems (Child-Pugh and Albumin-Bilirubin [ALBI]), and scanner characteristics were analyzed using uni- and multivariate analyses.
Results
There was a significant positive correlation between LSR and albumin (rho = 0.193; p < 0.001), platelet counts (rho = 0.148; p = 0.004), and sodium (rho = 0.161; p = 0.002); and a negative correlation between LSR and total bilirubin (rho = −0.215; p < 0.001) and AST (rho = −0.191; p < 0.001). Multivariate analysis confirmed independent significance for each of albumin (p = 0.022), total bilirubin (p = 0.045), AST (p = 0.031), platelet counts (p = 0.012), and sodium (p = 0.006). The presence of ascites (1.47 vs. 1.69, p < 0.001) and varices (1.55 vs. 1.69, p = 0.006) was related to significantly lower LSR. Similarly, patients with ALBI grade 1 had significantly higher LSR than patients with grade 2 (1.74 ± 0.447 vs. 1.56 ± 0.408, p < 0.001); and Child-Pugh A patients had a significantly higher LSR than Child-Pugh B (1.67 ± 0.44 vs. 1.49 ± 0.33, p = 0.021). Also, LSR was negatively correlated with MELD-Na scores (rho = −0.137; p = 0.013). However, one scanner brand was significantly associated with lower LSR (p < 0.001).
Conclusions
The liver enhancement on the hepatobiliary phase of gadoxetic acid–enhanced MRI is correlated with biomarkers of liver functions in a multicenter cohort. However, this correlation shows variations between scanner brands.
Key Points
• The correlation between liver enhancement on the hepatobiliary phase of gadoxetic acid–enhanced MRI and liver function is consistent in a multicenter-multivendor cohort.
• Signal intensity–based indices (liver-to-spleen ratio) can be used as an imaging biomarker of liver function.
• However, absolute values might change between vendors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gadoxetic acid is a hepatocellular specific contrast agent showing selective hepatocyte uptake, which peaks approximately 20 min after injection (hepatobiliary phase) [1]. Hepatobiliary phase images increase the detection rate of primary and secondary liver tumors by enhancing contrast differences between lesions and healthy liver tissue [2, 3]. Gadoxetic acid–enhanced MRI has been shown to improve treatment decisions based on the tumor extent compared to CT [4]. Furthermore, the degree of hepatic enhancement has been shown to be lower in patients with poor liver function. Previous studies have shown that hepatic enhancement is correlated with ALBI-score [5, 6], Child-Pugh grade [7], MELD-score [8], hypoalbuminemia [8,9,10], hyperbilirubinemia [9,10,11], INR [9], platelet count [9], serum sodium [8], and ascites [8]. However, all of these comprised single-center cohorts with a small number of patients. Additionally, the signal intensity of the liver is influenced by a variety of factors, including scanner type, field strength, and imaging sequence parameters [12]. Okada et al reported the first multicenter study on the correlation between biochemical parameters and hepatobiliary phase enhancement using the liver-to-spleen ratio (LSR) and showed that prothrombin activity, bilirubin, and total cholesterol levels are significantly associated with the liver enhancement in an Asian cohort [13]. There was no difference in signal intensity between 1.5- and 3.0-T scanners. Nonetheless, all patients within this study underwent MRI with machines from the same vendor. Recently, a single-center study showed that signal intensity (SI)–based indices (including LSR) remained similar when the same patient underwent consecutive MRI within the same center and identified no significant difference between scanners from the same vendor [14]. However, evaluation of the consistency of SI-based indices between different scanner brands lacks in the literature.
The objective of this post hoc study was to evaluate if the correlation between liver enhancement on gadoxetic acid MRI and liver function is preserved in a multivendor study cohort comprised of hepatocellular carcinoma (HCC) patients collected within a prospective randomized controlled trial (SORAMIC, SORAfenib in combination with local MICro-therapy guided by gadolinium-EOB-DTPA-enhanced MRI; EudraCT 2009-012576-27, NCT01126645) performed in multiple centers from various countries.
Material and methods
Study population
SORAMIC was a multicenter randomized-controlled phase II trial conducted at 38 sites in 12 countries. Within the palliative arm of the study, 424 patients with intermediate or advanced stage HCC (BCLC-B and C) were randomized either to sorafenib monotherapy or Y-90 radioembolization followed by sorafenib treatment between January 2011 and February 2016. Inclusion and exclusion criteria and results of the study were previously published [15]. In terms of liver functions, patients with Child-Pugh scores A to B7 were allowed to be included in the SORAMIC trial. For inclusion into this substudy, the presence of MRI with gadoxetic acid before randomization was necessary. The study protocol was approved by the institutional review boards of each participating center, and written informed consent was acquired from each patient, including a collection of baseline images for centralized analysis.
Of the 424 patients randomized within the SORAMIC trial, 372 patients had baseline MRI images, including the hepatobiliary phase, available. Four patients were excluded due to a history of splenectomy, eight patients due to missing baseline albumin or bilirubin values, and one patient due to significant artifacts precluding SI measurement in the spleen. The study population consisted of the remaining 359 patients from 31 centers out of eleven countries.
Imaging protocol
Patients underwent MRI with a standardized imaging protocol according to the diagnostic study of the SORAMIC trial [4]. Brands, models, field strengths of the scanners used in participating centers, and imaging parameters from exemplary centers recruited the highest number of patients for each scanner brand are listed in Supplementary tables 1 and 2. The MRI protocol consisted of pre-contrast T1-weighted gradient echo (GRE) sequences acquired 2D and 3D which was followed by an injection of 0.1 ml/kg gadoxetic acid and the dynamic series. At 20 min after contrast injection, T1-weighted GRE 2D and 3D hepatobiliary phase images were acquired. During the interval between dynamic series and hepatobiliary phase, T2-weighted turbo spin-echo 2D sequences and diffusion-weighted imaging (not mandatory) were performed.
Image analysis
Image analysis was performed by a radiologist with 6 years of experience in abdominal imaging who was blinded to all clinical parameters. SI of the liver and spleen on T1-weighted GRE 3D hepatobiliary phase images were calculated using a circular or oval ROI with a size of approximately 250 mm2. Large vessels, bile ducts, tumor lesions, and major artifacts were avoided during ROI placement. In order to sample the whole liver, two of the left liver lobe, right posterior sector, and right anterior sector were chosen (to exclude segments replaced with tumors). Then, the mean SI of the liver was measured separately three times in each of two, and the average SI was recorded for each measurement. LSR was calculated with the following formula:
Additionally, the presence of ascites, pleural effusion, and gastroesophageal varices in the images have been recorded (as present, not present).
Clinical laboratory parameters
All patients underwent clinical examination, complete blood count, and serum biochemical laboratory assessments at the participating centers before randomization. The diagnosis of cirrhosis was made by the local investigator based on the history, imaging, and clinical findings. The following parameters were included in this analysis: age, sex, body mass index (BMI), hemoglobin, white blood cell count, thrombocyte count, total bilirubin, albumin, alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), serum creatinine, and the international normalized ratio (INR). The Child-Pugh grade of the patients was also reported by centers. Additionally, MELD-Na and ALBI scores were calculated for each patient using the following formulas:
and MELD-Na score was rounded to nearest integer, and patients with an ALBI score of ≤ −2.60 were graded as 1, > −2.60 and ≤ −1.39 as 2, and > −1.39 as 3.
Statistical analysis
Analyses were performed using R statistical software (R version 3.6.3). Categorical variables were reported as counts and percentages, and continuous variables as means and standard deviations or medians and interquartile ranges. Univariate analyses for correlation of LSR with categorical variables were carried out using unpaired t-tests and one-way analysis of variance (ANOVA) tests. Non-parametric univariate correlation analysis (Spearman’s rank-order correlation) was performed for LSR and the clinical parameters. A p value < 0.05 was regarded as statistically significant. Variables with significant correlation were included in multivariate analysis. Patients with missing data were excluded from the multivariate analysis. We used the receiver operating characteristic (ROC) curve to determine the cut-off values for different scanners that could produce the highest sensitivity and specificity to predict higher ALBI grade.
Results
Baseline characteristics of the patients are summarized in Table 1. Out of 359 patients, 8 (2.2%) had initial (BCLC-A), 108 (30.1%) had intermediate (BCLC-B), and 243 (67.7%) had advanced (BCLC-C) HCC. Macrovascular invasion was present in 182 (50.6%) patients. A total of 286 (79.6%) patients had liver cirrhosis, 327 (91.1%) patients had Child-Pugh A liver functions, and 32 (8.9%) patients had B. Underlying liver disease was hepatitis B in 28 (10.6%), hepatitis C in 75 (24.4%), alcoholic liver disease in 125 (40.2%), hepatitis B and C in 1 (0.2%), hepatitis B and alcoholic liver disease in 8 (2.2%), and hepatitis C and alcoholic liver disease in 12 (3.3%), non-alcoholic fatty liver disease in 43 (11.9%), cryptogenic in 42 (11.6%), and hemochromatosis in 11 (3.0%) patients. Results of baseline laboratory values are summarized in Table 2. In total, 339 (94.4%) patients were imaged with a 1.5-T scanner and 20 (5.6%) patients with a 3-T.
Correlation of LSR with patient and tumor characteristics
Univariate analysis revealed no significant correlation between LSR and age (p = 0.186), body mass index (p = 0.288), and gender (p = 0.579).
Similarly, there was no significant correlation between LSR and the presence of macrovascular invasion (1.66 ± 0.48 vs. 1.65 ± 0.37, p = 0.853), BCLC stage (1.74 for A, 1.66 for B, and 1.65 for C; p = 0.825), and the ECOG performance score of patients (0 vs. ≥ 1; p = 0.773).
Correlation of LSR with laboratory parameters
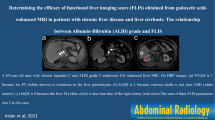
Results of the correlation analysis between LSR and laboratory parameters are listed in Table 3. Univariate analysis revealed a positive correlation between LSR and albumin (rho = 0.193; p < 0.001; Figure 1a), platelet counts (rho = 0.148; p = 0.004; Figure 1b), and sodium (rho = 0.161; p = 0.002; Figure 1c). In addition to this, there was a significant negative correlation between LSR and total bilirubin (rho = −0.215; p < 0.001; Figure 1d) and AST (rho = −0.191; p < 0.001; Figure 1e).
Correlation between LSR and biochemical parameters. Scatterplots with linear regression lines showing LSR versus with the variables with statistical significance: a Albumin (rho = 0.193, p < 0.001), (b) total bilirubin (rho = −0.215, p < 0.001), (c) AST (rho = −0.191, p < 0.001), (d) sodium (rho = 0.161, p = 0.002), (e) platelet count (rho = 0.148, p = 0.004), and (f) ALBI score (rho = −0.225, p < 0.001). ALBI, albumin-bilirubin; AST, aspartate transaminase; LSR, liver-to-spleen ratio
Multiple regression analysis with 335 patients confirmed the significant correlation between LSR and albumin (p = 0.033), total bilirubin (p = 0.034), AST (p = 0.024), platelet counts (p = 0.009), and sodium (p = 0.004).
Correlation of LSR with clinical features related to liver functions
The presence of cirrhosis was significantly correlated with lower LSR (1.62 ± 0.43 vs. 1.77 ± 0.4, p = 0.009). Ascites was present in 61 (16.9%), pleural effusion in 11 (3.0%), and varices in 98 (27.2%) patients. Patients with ascites had significantly lower LSR than patients without (1.69 vs. 1.47, p < 0.001), as well as varices (1.69 vs. 1.55, p = 0.006). Although the patients with pleural effusion had lower LSR, the difference was marginally non-significant (1.66 vs. 1.39, p = 0.056).
Correlation of LSR with liver function scoring systems
There was a significant negative correlation between the LSR and ALBI scores (rho = −0.225; p < 0.001; Figure 1f), and patients with ALBI grade 1 had significantly higher LSR than patients with grade 2 (1.74 ± 0.447 vs. 1.56 ± 0.408, p < 0.001). Similarly, there was a significant negative correlation between LSR and MELD-Na scores (rho = −0.137; p = 0.013). Patients with Child-Pugh score A had significantly higher LSR than patients with Child-Pugh B (1.67 ± 0.44 vs. 1.49 ± 0.33, p = 0.021).
Correlation of LSR with scanner characteristics
There was no significant difference in LSR between patients who underwent MRI with 1.5-T and 3-T scanners (1.65 ± 0.43 vs. 1.74 ± 0.45, p = 0.853).
However, patients scanned with one scanner brand (Philips) had significantly lower LSR than all the other three brands (p < 0.001; Figure 2). In order to eliminate possible differences in liver functions between scanner brands, a separate multivariate model was created including scanner brand in addition to parameters having a statistically significant correlation with LSR. This model confirmed that scanner brand is independently associated with LSR, and other parameters preserved significant correlation with LSR as in the initial model (Supplementary table 3). There was no significant difference between the other brands. By ROC curve analysis revealed a cut-off value of 1.46 for LSR to have the highest sensitivity (55.2%) and specificity (67.1%) to predict ALBI grade ≥ 2 for Philips scanners, and 1.66 with a sensitivity of 57.9% and specificity of 63.1% for other scanners (Supplementary figure 1).
Discussion
Our results show that LSR, a hepatobiliary phase MRI SI-based parameter, maintains the correlation with biochemical parameters of liver function and liver function grading systems in a large cohort collected within a multicenter, multivendor study. This finding supports the routine clinical usage of hepatobiliary phase images as an imaging marker of liver functions. In addition to laboratory parameters, liver enhancement on the hepatobiliary phase could be used as a surveillance parameter of liver parenchymal functional status in patients with cirrhosis. Additionally, it could serve in the complex decision-making process of HCC treatment, especially as a decision aid in favor of resection or local ablation in the early stage of HCC disease by predicting post-hepatectomy liver failure with estimation of the functional reserve capacity. However, although this correlation is preserved between scanners, absolute LSR values differ between vendors. One brand had significantly lower LSR values than other brands, and this difference was preserved in a multivariable analysis considering other biochemical parameters correlated with LSR.
There was a significant correlation between LSR and several clinical laboratory parameters in our study, including albumin, total bilirubin, AST, platelet count, and sodium. LSR was also correlated with imaging findings related to deteriorated liver functions, such as ascites and varices. All of these parameters are known to correlate with liver function and incorporated in survival prediction systems in patients with chronic liver diseases [16,17,18,19,20,21]. Furthermore, LSR had a significant correlation with the two most commonly used liver function grading systems [18, 19, 22].
Gadoxetic acid has an intra- and extravascular compartment distribution in arterial and portal venous phases, similar to other gadolinium-based contrast media; however, it is actively taken up by hepatocytes via organic anion transporting polypeptides (OATP1B1/3) during the transitional and hepatobiliary phases [23]. Increased contrast between lesions and parenchyma in the hepatobiliary phase has improved treatment decisions in primary and secondary liver tumors [2, 4, 24, 25]. Liver enhancement on the hepatobiliary phase depends on the concentration of OATPs in the liver, which has been shown to decrease as fibrosis progresses, as well as the total number of hepatocytes [26]. Additionally, Na+ - taurocholate cotransporting polypeptide (NTCP), which influxes two sodium ions and one conjugated bile salt into hepatocytes, is shown to uptake gadoxetic acid with higher affinity but lower capacity than OATPs [27]. By these features, gadoxetic acid–enhanced MRI offers the possibility to evaluate liver functions with additional advantages over biochemical markers or other imaging-based assessments, including the possibility to assess regional liver functions [28]. However, SI measurements are relative parameters that depend on many technical parameters; therefore, these values cannot be used to compare consequent studies of the patient or different patients. Several SI-based parameters have been described to overcome these variations by correcting liver enhancement with spleen or muscle intensities. Previous studies have shown a good to a perfect positive correlation between LSR, relative liver enhancement, contrast uptake index, and hepatocyte uptake index in patients with chronic liver disease (0.715–0.906), as well as a good to a perfect intra- and interobserver correlation for each parameter [6].
Several studies have shown that these parameters are significantly correlated with biochemical markers of liver function, the presence of liver disease, the degree of liver fibrosis, and liver function scoring systems [5, 7,8,9]. However, most of these studies were single-center retrospective cohorts with a limited number of patients. A multicenter study performed in Japan using the same scanner brand showed that sufficient liver enhancement (LSR > 1.5) is correlated with prothrombin activity, total bilirubin, and total cholesterol levels [13]. More recently, a single-center study showed that liver enhancement parameters (LSR, relative enhancement, liver-to-muscle ratio) are consistent between consecutive imaging with no difference between scanners using different field strengths from the same brand [14]. Our study confirmed the correlation between signal intensity-based assessments (LSR) and liver function in a multicenter, multivendor study using a much broader variability in scanner brands and field strengths. However, probably due to variations and heterogeneities between centers, scanner brands, and different etiologies of underlying liver diseases, the correlation was weaker than previously published results. For example, the correlation coefficient of LSR with ALBI score was −0.225 in our study, while Takatsu et al and Beer et al reported −0.61 and −0.491 [5, 6].
Additionally, our study showed that despite the significant correlation between LSR and liver function tests, absolute LSR values differ between vendors. Patients who underwent MRI with one vendor showed significantly lower LSR than other brands, and this difference was confirmed in a separate multivariate analysis considering liver function. We evaluated imaging parameters for scanner brands, and despite slight variations, we believe the difference in absolute LSR values results from different post-processing software. However, it did not affect the correlation with liver function parameters as described above. This indicates that the scanner brand should be taken into account when absolute levels are used in decision making, such as sufficient liver enhancement (LSR > 1.5). Additionally, explorative ROC analysis to define cut-off values for LSR discriminating ALBI grade identified 1.46 for Philips and 1.66 for other brands to have the highest sensitivity and specificity. Another interesting finding in our study was the significant correlation between LSR and serum sodium levels. Although hyponatremia is common at advanced stages of cirrhosis and incorporated into the MELD-Na score, a decrease in NCTP activity in patients with low serum sodium might also be contributing and requires further analysis.
HCC was the fourth common cause of cancer-related death worldwide in 2018 [29], and its incidence is expected to increase, especially in Western countries [30]. In the Western population, up to 90% of the cases develop within the background of chronic liver disease, and besides tumor extent, the liver function of the patient is the primary determinant of outcome [31]. Several staging systems have been developed to predict outcomes and plan optimal treatment, and all have incorporated various parameters related to the liver function of the patient [32,33,34]. Gadoxetic acid MRI provides better treatment allocation than CT considering tumor burden [4], and, in addition to this, information related to liver function. It has been shown that liver function assessment by gadoxetic acid–enhanced MRI can predict post-hepatectomy liver failure [35, 36], can be used to plan hepatectomy by evaluation of regional liver function [37], and is superior to the indocyanine clearance test in predicting complications [38]. Additionally, some novel imaging parameters related to tumor biology (i.e., presence of peritumoral hypointensity) can be evaluated on hepatobiliary phase images [39]. As a result of these advantages, gadoxetic acid–enhanced MRI has been the primary evaluation tool in HCC patients [40]. Our research adds further evidence to the implementation of gadoxetic acid–derived liver function assessment in patients with hepatocellular carcinoma [41].
Our study has several limitations. First, despite the standardized MRI imaging protocol within the prospective imaging study, there were variations in imaging parameters (TE, FA, fat-saturation techniques) at centers. However, all centers obtained the 3D T1-weighted fat-sat sequences with a fixed time delay for the hepatobiliary phase. Further on, liver function assessment was based on a relative parameter using the ratio of liver and spleen measurements obtained at the same phase and same slice, which should be robust against these variabilities. Second, ROI placement may have caused variations related to the heterogeneity of liver functions in different segments. To overcome this limitation, multiple ROIs were placed at different liver segments to increase sampling volume, and there was a perfect correlation (rho = 0.975) between two measurements obtained within the liver (not shown). Third, all patients had relatively preserved liver function with no patients having Child-Pugh class C, which narrows the range of LSR in our cohort and limits to evaluate changes in liver enhancement in patients with worse liver functions. Also, there was only one reader, and only one of the signal intensity-based indices (LSR) was used. However, LSR was one of the most commonly used parameters and is robust to calculate, with measurements from liver and spleen done within the same slice (unlike liver-to-muscle ratio), and as pointed out above, previous studies have shown perfect intra- and interobserver correlation of LSR [6]. Finally, the number of patients imaged with 3.0T scanners was low (n = 20), and we did not find any difference in LSR between patients scanned with different field strengths. Although the data is not sufficiently powered to make a statement on the field strength, this result was in agreement with previous reports [13]. Additionally, this analysis aimed to confirm if the correlation is preserved in a multivendor cohort instead of comparing the different parameters of a MRI-based liver function assessment.
In conclusion, the correlation of liver enhancement on the hepatobiliary phase of gadoxetic acid–enhanced MRI with liver function is maintained between several centers and field strengths in patients with HCC, with or without underlying liver cirrhosis. This underlines the importance of gadoxetic acid–enhanced MRI in the treatment decision-making process as an imaging marker of liver functions.
Abbreviations
- ALBI:
-
Albumin-bilirubin
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- BCLC:
-
Barcelona clinic liver cancer
- BMI:
-
Body-mass index
- GGT:
-
Gamma-glutamyl transpeptidase
- GRE:
-
Gradient echo
- INR:
-
International normalized ratio
- LSR:
-
Liver-to-spleen ratio
- MELD:
-
Model of end-stage liver disease
- OATP:
-
Organic anion transporting polypeptides
References
Motosugi U, Ichikawa T, Tominaga L et al (2009) Delay before the hepatocyte phase of Gd-EOB-DTPA-enhanced MR imaging: is it possible to shorten the examination time? Eur Radiol 19:2623–2629
Hayoz R, Vietti-Violi N, Duran R, Knebel JF, Ledoux JB, Dromain C (2020) The combination of hepatobiliary phase with Gd-EOB-DTPA and DWI is highly accurate for the detection and characterization of liver metastases from neuroendocrine tumor. Eur Radiol 30:6593–6602
Karaosmanoglu AD, Onur MR, Ozmen MN, Akata D, Karcaaltincaba M (2016) Magnetic Resonance imaging of liver metastasis. Semin Ultrasound CT MR 37:533–548
Ricke J, Steffen IG, Bargellini I et al (2020) Gadoxetic acid-based hepatobiliary MRI in hepatocellular carcinoma. JHEP Rep 2:100173
Takatsu Y, Kobayashi S, Miyati T, Shiozaki T (2016) Hepatobiliary phase images using gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced MRI as an imaging surrogate for the albumin-bilirubin grading system. Eur J Radiol 85:2206–2210
Beer L, Mandorfer M, Bastati N et al (2019) Inter- and intra-reader agreement for gadoxetic acid-enhanced MRI parameter readings in patients with chronic liver diseases. Eur Radiol 29:6600–6610
Motosugi U, Ichikawa T, Sou H et al (2009) Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement? J Magn Reson Imaging 30:1042–1046
Kim HY, Choi JY, Park CH et al (2013) Clinical factors predictive of insufficient liver enhancement on the hepatocyte-phase of Gd-EOB-DTPA-enhanced magnetic resonance imaging in patients with liver cirrhosis. J Gastroenterol 48:1180–1187
Matsushima S, Sato Y, Yamaura H et al (2014) Visualization of liver uptake function using the uptake contrast-enhanced ratio in hepatobiliary phase imaging. Magn Reson Imaging 32:654–659
Matoori S, Froehlich JM, Breitenstein S et al (2019) Serum albumin, total bilirubin, and patient age are independent confounders of hepatobiliary-phase gadoxetate parenchymal liver enhancement. Eur Radiol 29:5813–5822
Higaki A, Tamada T, Sone T et al (2012) Potential clinical factors affecting hepatobiliary enhancement at Gd-EOB-DTPA-enhanced MR imaging. Magn Reson Imaging 30:689–693
Onoda M, Hyodo T, Murakami T et al (2015) Optimizing signal intensity correction during evaluation of hepatic parenchymal enhancement on gadoxetate disodium-enhanced MRI: comparison of three methods. Eur J Radiol 84:339–345
Okada M, Murakami T, Kuwatsuru R et al (2016) Biochemical and clinical predictive approach and time point analysis of hepatobiliary phase liver enhancement on Gd-EOB-DTPA-enhanced MR images: a multicenter study. Radiology 281:474–483
Theilig D, Elkilany A, Schmelzle M et al (2019) Consistency of hepatocellular gadoxetic acid uptake in serial MRI examinations for evaluation of liver function. Abdom Radiol (NY) 44:2759–2768
Ricke J, Klümpen HJ, Amthauer H et al (2019) Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 71:1164–1174
Wang Y, Zhong J, Su Z et al (2016) Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 103:725–734
Londoño M-C, Cárdenas A, Guevara M et al (2007) MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut 56:1283–1290
Liu PH, Hsu CY, Hsia CY et al (2017) ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol 32:879–886
Johnson PJ, Berhane S, Kagebayashi C et al (2015) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—the ALBI grade. J Clin Oncol 33:550
Durand F, Valla D (2005) Assessment of the prognosis of cirrhosis: Child–Pugh versus MELD. J Hepatol 42:S100–S107
Biggins SW, Kim WR, Terrault NA et al (2006) Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 130:1652–1660
Albers I, Hartmann H, Bircher J, Creutzfeldt W (1989) Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol 24:269–276
Tsuboyama T, Onishi H, Kim T et al (2010) Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging--correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology 255:824–833
Morin C, Drolet S, Daigle C et al (2020) Additional value of gadoxetic acid-enhanced MRI to conventional extracellular gadolinium-enhanced MRI for the surgical management of colorectal and neuroendocrine liver metastases. HPB (Oxford) 22:710–715
Puhr-Westerheide D, Cyran CC, Sargsyan-Bergmann J et al (2019) The added diagnostic value of complementary gadoxetic acid-enhanced MRI to (18)F-DOPA-PET/CT for liver staging in medullary thyroid carcinoma. Cancer Imaging 19:73
Verloh N, Probst U, Utpatel K et al (2019) Influence of hepatic fibrosis and inflammation: correlation between histopathological changes and Gd-EOB-DTPA-enhanced MR imaging. PLoS One 14:e0215752
Leonhardt M, Keiser M, Oswald S et al (2010) Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos 38:1024–1028
Merkle EM, Zech CJ, Bartolozzi C et al (2016) Consensus report from the 7th International Forum for Liver Magnetic Resonance Imaging. Eur Radiol 26:674–682
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS (2016) Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol 34:1787–1794
Vogel A, Cervantes A, Chau I et al (2018) Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv238–iv255
Llovet JM, Brú C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19:329–338
Okuda K, Ohtsuki T, Obata H et al (1985) Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 56:918–928
Investigators CLIP (1998) A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology 28:751–755
Jin YJ, Lee SH, Cho SG et al (2016) Prediction of postoperative liver failure using gadoxetic acid-enhanced magnetic resonance imaging in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 31:1349–1356
Tsujita Y, Sofue K, Komatsu S et al (2020) Prediction of post-hepatectomy liver failure using gadoxetic acid-enhanced magnetic resonance imaging for hepatocellular carcinoma with portal vein invasion. Eur J Radiol 130:109189
Araki K, Harimoto N, Yamanaka T et al (2020) Efficiency of regional functional liver volume assessment using Gd-EOB-DTPA-enhanced magnetic resonance imaging for hepatocellular carcinoma with portal vein tumor thrombus. Surg Today 50:1496–1506
Kim DK, Choi JI, Choi MH et al (2018) Prediction of posthepatectomy liver failure: MRI with hepatocyte-specific contrast agent versus indocyanine green clearance test. AJR Am J Roentgenol 211:580–587
Kim AY, Sinn DH, Jeong WK et al (2018) Hepatobiliary MRI as novel selection criteria in liver transplantation for hepatocellular carcinoma. J Hepatol 68:1144–1152
Zech CJ, Ba-Ssalamah A, Berg T et al (2020) Consensus report from the 8th International Forum for Liver Magnetic Resonance Imaging. Eur Radiol 30:370–382
Poetter-Lang S, Bastati N, Messner A et al (2020) Quantification of liver function using gadoxetic acid-enhanced MRI. Abdom Radiol (NY) 45:3532–3544
Funding
Open Access funding enabled and organized by Projekt DEAL. SORAMIC is an investigator-initiated trial sponsored by the University of Magdeburg. Financial support was granted by Sirtex Medical and Bayer Healthcare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Max Seidensticker.
Conflict of interest
Bernhard Gebauer: Personal fees: Bayer, Sirtex.
Irene Bargellini: Grants: Sirtex, during the conduct of the study. Personal fees: Bayer, Sirtex, Biocompatibles, Terumo, outside the submitted work.
Maciej Pech: Grants: Sirtex, Bayer; Personal fees: Sirtex.
Peter Malfertheiner: Grants: Bayer, Sirtex.
Jens Ricke: Grants: Sirtex, Bayer; Personal fees: Sirtex, Bayer.
Max Seidensticker: Personal fees: Bayer, Sirtex
Statistics and biometry
One of the authors (OÖ) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap:
The main results of the clinical trial have been previously reported in Ricke J Hepatology 2019.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 225 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Öcal, O., Peynircioglu, B., Loewe, C. et al. Correlation of liver enhancement in gadoxetic acid–enhanced MRI with liver functions: a multicenter-multivendor analysis of hepatocellular carcinoma patients from SORAMIC trial. Eur Radiol 32, 1320–1329 (2022). https://doi.org/10.1007/s00330-021-08218-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08218-9