Abstract
Objective
To systematically investigate the effect of imaging features at different DCE-MRI phases to optimise a radiomics model based on DCE-MRI for the prediction of tumour-infiltrating lymphocyte (TIL) levels in breast cancer.
Materials and methods
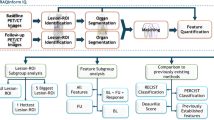
This study retrospectively collected 133 patients with pathologically proven breast cancer, including 73 patients with low TIL levels and 60 patients with high TIL levels. The volumes of breast cancer lesions were manually delineated on T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and each phase of DCE-MRI, followed by 6250 quantitative feature extractions. The least absolute shrinkage and selection operator (LASSO) method was used to select predictive feature sets for the classifiers. Four models were developed for predicting TILs: (1) single enhanced phase radiomics models; (2) fusion enhanced multi-phase radiomics models; (3) fusion multi-sequence radiomics models; and (4) a combined radiomics-based clinical model.
Results
Image features extracted from the delayed phase MRI, especially DCE_Phase 6 (DCE_P6), demonstrated dominant predictive performances over features from other phases. The fusion multi-sequence radiomics model and combined radiomics-based clinical model achieved the highest predictive performances with areas under the curve (AUCs) of 0.934 and 0.950, respectively; however, the differences were not statistically significant.
Conclusion
The DCE-MRI radiomics model, especially image features extracted from the delayed phases, can help improve the performance in predicting TILs. The radiomics nomogram is effective in predicting TILs in breast cancer.
Key Points
• Radiomics features extracted from DCE-MRI, especially delayed phase images, help predict TIL levels in breast cancer.
• We developed a nomogram based on MRI to predict TILs in breast cancer that achieved the highest AUC of 0.950.







Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- DCE:
-
Dynamic contrast-enhanced
- DCE_P6:
-
DCE_Phase 6
- DWI:
-
Diffusion-weighted imaging
- ER:
-
Estrogen receptor
- GLCM:
-
Gray-level co-occurrence matrix
- GLRLM:
-
Gray-level run-length matrix
- GLSZM:
-
Gray-level size-zone matrix
- HER-2:
-
Human epidermal growth factor receptor-2
- ICC:
-
Interobserver correlation coefficient
- LASSO:
-
Least absolute shrinkage and selection operator
- MRI:
-
Magnetic resonance imaging
- NAC:
-
Neo-adjuvant chemotherapy
- NGTDM:
-
Neighborhood gray tone difference matrix
- PR:
-
Progesterone receptor
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- T2WI:
-
T2-weighted imaging
- TIL:
-
Tumour-infiltrating lymphocyte
References
Savas P, Salgado R, Denkert C et al (2016) Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 13:228–241
Ingold Heppner B, Untch M, Denkert C et al (2016) Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res 22:5747–5754
Denkert C, Wienert S, Poterie A et al (2016) Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol 29:1155–1164
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271
Dieci MV, Radosevic-Robin N, Fineberg S et al (2018) Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol 52:16–25
Bellesoeur A, Torossian N, Amigorena S, Romano E (2020) Advances in theranostic biomarkers for tumor immunotherapy. Curr Opin Chem Biol 56:79–90
Dromain C, Beigelman C, Pozzessere C, Duran R, Digklia A (2020) Imaging of tumour response to immunotherapy. Eur Radiol Exp 4:2
Celebi F, Agacayak F, Ozturk A et al (2020) Usefulness of imaging findings in predicting tumor-infiltrating lymphocytes in patients with breast cancer. Eur Radiol 30:2049–2057
Ku YJ, Kim HH, Cha JH et al (2018) Predicting the level of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: usefulness of breast MRI computer-aided detection and diagnosis. J Magn Reson Imaging 47:760–766
Fogante M, Tagliati C, De Lisa M, Berardi R, Giuseppetti GM, Giovagnoni A (2019) Correlation between apparent diffusion coefficient of magnetic resonance imaging and tumor-infiltrating lymphocytes in breast cancer. Radiol Med 124:581–587
W-j T, Jin Z, Y-l Z et al (2021) Whole-lesion histogram analysis of the apparent diffusion coefficient as a quantitative imaging biomarker for assessing the level of tumor-infiltrating lymphocytes: value in molecular subtypes of breast cancer. Front Oncol 10:611571
Murakami W, Tozaki M, Sasaki M et al (2020) Correlation between (18)F-FDG uptake on PET/MRI and the level of tumor-infiltrating lymphocytes (TILs) in triple-negative and HER2-positive breast cancer. Eur J Radiol 123:108773
Cook G, Goh V (2020) A role for FDG PET radiomics in personalized medicine? Semin Nucl Med 50:532–540
Ma W, Ji Y, Qi L, Guo X, Jian X, Liu P (2018) Breast cancer Ki67 expression prediction by DCE-MRI radiomics features. Clin Radiol 73:909.e901–909.e905
Fan M, Yuan W, Zhao W et al (2020) Joint prediction of breast cancer histological grade and Ki-67 expression level based on DCE-MRI and DWI radiomics. IEEE J Biomed Health Inform 24:1632–1642
Liu C, Ding J, Spuhler K et al (2019) Preoperative prediction of sentinel lymph node metastasis in breast cancer by radiomic signatures from dynamic contrast-enhanced MRI. J Magn Reason Imaging 49:131–140
Liu J, Sun D, Chen L et al (2019) Radiomics analysis of dynamic contrast-enhanced magnetic resonance imaging for the prediction of sentinel lymph node metastasis in breast cancer. Front Oncol 9:980
Braman N, Etesami M, Prasanna P et al (2017) Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res 19:57
Liu Z, Li Z, Qu J et al (2019) Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res 25:3538–3547
Liu Z, Feng B, Li C et al (2019) Preoperative prediction of lymphovascular invasion in invasive breast cancer with dynamic contrast-enhanced-MRI-based radiomics. J Magn Reason Imaging 50:847–857
Denkert C, von Minckwitz G, Darb-Esfahani S et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19:40–50
Aerts H, Velazquez E, Leijenaar R et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Wang J, Kato F, Oyama-Manabe N et al (2015) Identifying triple-negative breast cancer using background parenchymal enhancement heterogeneity on dynamic contrast-enhanced MRI: a pilot radiomics study. PLoS One 10:e0143308
Burugu S, Asleh-Aburaya K, Nielsen T (2017) Immune infiltrates in the breast cancer microenvironment: detection, characterization and clinical implication. Breast Cancer 24:3–15
Jones E, Sinha S, Newitt D et al (2013) MRI enhancement in stromal tissue surrounding breast tumors: association with recurrence free survival following neoadjuvant chemotherapy. PLoS One 8:e61969
Wegner C, Gaustad J, Andersen L, Simonsen T, Rofstad E (2016) Diffusion-weighted and dynamic contrast-enhanced MRI of pancreatic adenocarcinoma xenografts: associations with tumor differentiation and collagen content. J Transl Med 14:161
Cao J, Pickup S, Clendenin C et al (2019) Dynamic contrast-enhanced MRI detects responses to stroma-directed therapy in mouse models of pancreatic ductal adenocarcinoma. Clin Cancer Res 25:2314–2322
Asayama Y, Yoshimitsu K, Irie H et al (2006) Delayed-phase dynamic CT enhancement as a prognostic factor for mass-forming intrahepatic cholangiocarcinoma. Radiology 238:150–155
Lacomis J, Baron R, Oliver J, Nalesnik M, Federle M (1997) Cholangiocarcinoma: delayed CT contrast enhancement patterns. Radiology 203:98–104
Stanton S, Disis M (2016) Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer 4:59
Saltz J, Gupta R, Hou L et al (2018) Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell Rep 23:181–193.e187
Song Q, Shi F, Adair M et al (2019) Cell counts, rather than proportion, of CD8/PD-1 tumor-infiltrating lymphocytes in a tumor microenvironment associated with pathological characteristics of Chinese invasive ductal breast cancer. J Immunol Res 2019:8505021
Huang W, Ran R, Shao B, Li H (2019) Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast Cancer Res Treat 178:17–33
Kim S, Jeong H, Woo O et al (2013) Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol 36:224–231
Solinas C, Carbognin L, De Silva P, Criscitiello C, Lambertini M (2017) Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: current state of the art. Breast 35:142–150
Wein L, Savas P, Luen SJ, Virassamy B, Salgado R, Loi S (2017) Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol 7:156
Acknowledgements
We gratefully acknowledge all the members of the Department of Radiology, Guangzhou First People’s Hospital.
Funding
This study has received funding from the National Natural Science Foundation of China (No. 81901711).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Xin-qing Jiang.
Conflict of interest
The authors declare no competing interests.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• experimental
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 728 kb)
Rights and permissions
About this article
Cite this article
Tang, Wj., Kong, Qc., Cheng, Zx. et al. Performance of radiomics models for tumour-infiltrating lymphocyte (TIL) prediction in breast cancer: the role of the dynamic contrast-enhanced (DCE) MRI phase. Eur Radiol 32, 864–875 (2022). https://doi.org/10.1007/s00330-021-08173-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08173-5