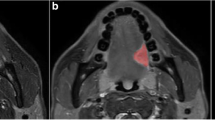
Abstract
Objective
Discriminating metastatic from benign cervical lymph nodes (LNs) in oral squamous cell carcinoma (OSCC) patients using pretreatment computed tomography (CT) has been controversial. This study aimed to investigate whether CT-based texture analysis with machine learning can accurately identify cervical lymph node metastasis in OSCC patients.
Methods
Twenty-three patients (with 201 cervical LNs [150 benign, 51 metastatic] at levels I–V) who underwent preoperative contrast-enhanced CT and subsequent cervical neck dissection were enrolled. Histopathologically proven LNs were randomly divided into the training cohort (70%; n = 141, at levels I–V) and validation cohort (30%; n = 60, at level I/II). Twenty-five texture features and the nodal size of targeted LNs were analyzed on the CT scans. The nodal-based sensitivities, specificities, diagnostic accuracy rates, and the area under the curves (AUCs) of the receiver operating characteristic curves of combined features using a support vector machine (SVM) at levels I/II, I, and II were evaluated and compared with two radiologists and a dentist (readers).
Results
In the validation cohort, the AUCs (0.820 at level I/II, 0.820 at level I, and 0.930 at level II, respectively) of the radiomics approach were superior to three readers (0.798–0.816, 0.773–0.798, and 0.825–0.865, respectively). The best models were more specific at levels I/II and I and accurate at each level than each of the readers (p < .05).
Conclusions
Machine learning–based analysis with contrast-enhanced CT can be used to noninvasively differentiate between benign and metastatic cervical LNs in OSCC patients.
Key Points
• The best algorithm in the validation cohort can noninvasively differentiate between benign and metastatic cervical LNs at levels I/II, I, and II.
• The AUCs of the model at each level were superior to those of multireaders.
• Significant differences in the specificities at level I/II and I and diagnostic accuracy rates at each level between the model and multireaders were found.



Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- FDG:
-
2-[18F]Fluoro-2-deoxy-D-glucose
- GLCM:
-
Gray-level co-occurrence matrix features
- GLZLM:
-
Gray-level zone length matrix
- LN:
-
Cervical lymph node
- OSCC:
-
Oral squamous cell carcinoma
- PET-CT:
-
Positron emission tomography-computed tomography
- RFE:
-
Recursive feature elimination
- ROI:
-
Region of interest
- SVM:
-
Support vector machine
References
Snow GB, Patel P, Leemans CR, Tiwari R (1992) Management of cervical lymph nodes in patients with head and neck cancer. Eur Arch Otorhinolaryngol 249:187–194
Matsubara R, Kawano S, Chikui T et al (2012) Clinical significance of combined assessment of the maximum standardized uptake value of F-18 FDG PET with nodal size in the diagnosis of cervical lymph node metastasis of oral squamous cell carcinoma. Acad Radiol 19:708–717. https://doi.org/10.1016/j.acra.2012.02.009
Park JT, Roh JL, Kim JS et al (2016) (18)F FDG PET/CT versus CT/MR imaging and the prognostic value of contralateral neck metastases in patients with head and neck squamous cell carcinoma. Radiology 279:481–491. https://doi.org/10.1148/radiol.2015150959
Shin NY, Lee JH, Kang WJ, Koh YW, Sohn B, Kim J (2015) Clinical usefulness of [18F]FDG PET-CT and CT/MRI for detecting nodal metastasis in patients with hypopharyngeal squamous cell carcinoma. Ann Surg Oncol 22:994–999. https://doi.org/10.1245/s10434-014-4062-2
Sumi M, Ohki M, Nakamura T (2001) Comparison of sonography and CT for differentiating benign from malignant cervical lymph nodes in patients with squamous cell carcinoma of the head and neck. AJR Am J Roentgenol 176:1019–1024. https://doi.org/10.2214/ajr.176.4.1761019
Sun R, Tang X, Yang Y, Zhang C (2015) (18)FDG-PET/CT for the detection of regional nodal metastasis in patients with head and neck cancer: a meta-analysis. Oral Oncol 51:314–320. https://doi.org/10.1016/j.oraloncology.2015.01.004
Yamazaki Y, Saitoh M, Notani K et al (2008) Assessment of cervical lymph node metastases using FDG-PET in patients with head and neck cancer. Ann Nucl Med 22:177–184. https://doi.org/10.1007/s12149-007-0097-9
Takamochi K, Yoshida J, Murakami K et al (2005) Pitfalls in lymph node staging with positron emission tomography in non-small cell lung cancer patients. Lung Cancer 47:235–242.
Kitajima K, Suenaga Y, Minamikawa T et al (2015) Clinical significance of SUVmax in (18)F-FDG PET/CT scan for detecting nodal metastases in patients with oral squamous cell carcinoma. Springerplus 4:718-015-1521-6. eCollection 2015. https://doi.org/10.1186/s40064-015-1521-6
Robbins KT, Clayman G, Levine PA et al (2002) Neck dissection classification update: Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg 128:751–758 ooa20089 [pii]
Ganeshan B, Goh V, Mandeville HC, Ng QS, Hoskin PJ, Miles KA (2013) Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 266:326–336. https://doi.org/10.1148/radiol.12112428
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22:796–802. https://doi.org/10.1007/s00330-011-2319-8
Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K (2012) Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol 67:157–164. https://doi.org/10.1016/j.crad.2011.08.012
Zhang H, Graham CM, Elci O et al (2013) Locally advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated with induction chemotherapy. Radiology 269:801–809. https://doi.org/10.1148/radiol.13130110
Kuno H, Garg N, Qureshi M et al (2019) CT texture analysis of cervical lymph nodes on contrast-enhanced [18F] FDG-PET/CT images to differentiate nodal metastases from reactive lymphadenopathy in HIV-positive patients with head and neck squamous cell carcinoma. AJNR Am J Neuroradiol 40:543–550. https://doi.org/10.3174/ajnr.A5974
Kuno H, Qureshi MM, Chapman MN et al (2017) CT texture analysis potentially predicts local failure in head and neck squamous cell carcinoma treated with chemoradiotherapy. AJNR Am J Neuroradiol 38:2334–2340. https://doi.org/10.3174/ajnr.A5407
Kim H, Park CM, Park SJ et al (2016) Temporal changes of texture features extracted from pulmonary nodules on dynamic contrast-enhanced chest computed tomography: how influential is the scan delay? Invest Radiol 51:569–574. https://doi.org/10.1097/RLI.0000000000000267
Andersen MB, Harders SW, Ganeshan B, Thygesen J, Torp Madsen HH, Rasmussen F (2016) CT texture analysis can help differentiate between malignant and benign lymph nodes in the mediastinum in patients suspected for lung cancer. Acta Radiol 57:669–676. https://doi.org/10.1177/0284185115598808
Agner SC, Rosen MA, Englander S et al (2014) Computerized image analysis for identifying triple-negative breast cancers and differentiating them from other molecular subtypes of breast cancer on dynamic contrast-enhanced MR images: a feasibility study. Radiology 272:91–99. https://doi.org/10.1148/radiol.14121031
Ji GW, Zhang YD, Zhang H et al (2019) Biliary tract cancer at CT: a radiomics-based model to predict lymph node metastasis and survival outcomes. Radiology 290:90–98. https://doi.org/10.1148/radiol.2018181408
Truhn D, Schrading S, Haarburger C, Schneider H, Merhof D, Kuhl C (2019) Radiomic versus convolutional neural networks analysis for classification of contrast-enhancing lesions at multiparametric breast MRI. Radiology 290:290–297. https://doi.org/10.1148/radiol.2018181352
Nioche C, Orlhac F, Boughdad S et al (2018) LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 78:4786–4789. https://doi.org/10.1158/0008-5472.CAN-18-0125
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Mannil M, von Spiczak J, Manka R, Alkadhi H (2018) Texture analysis and machine learning for detecting myocardial infarction in noncontrast low-dose computed tomography: unveiling the invisible. Invest Radiol 53:338-343. https://doi.org/10.1097/RLI.0000000000000448
Yang L, Dong D, Fang M et al (2018) Can CT-based radiomics signature predict KRAS/NRAS/BRAF mutations in colorectal cancer? Eur Radiol 28:2058–2067. https://doi.org/10.1007/s00330-017-5146-8
Feng Z, Rong P, Cao P et al (2018) Machine learning-based quantitative texture analysis of CT images of small renal masses: differentiation of angiomyolipoma without visible fat from renal cell carcinoma. Eur Radiol 28:1625–1633. https://doi.org/10.1007/s00330-017-5118-z
Ng SH, Yen TC, Liao CT et al (2005) 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: a prospective study of 124 patients with histologic correlation. J Nucl Med 46:1136–1143
Bayanati H, Thornhill ER, Souza CA et al (2015) Quantitative CT texture and shape analysis: can it differentiate benign and malignant mediastinal lymph nodes in patients with primary lung cancer? Eur Radiol 25:480–487. https://doi.org/10.1007/s00330-014-3420-6
Davnall F, Yip CS, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589. https://doi.org/10.1007/s13244-012-0196-6
Acknowledgements
The present study was supported by a grant from St. Marianna University School of Medicine (St. Marianna University School of Medicine Research Grant).
Funding
The present study was supported by a grant from St. Marianna University School of Medicine (St. Marianna University School of Medicine Research Grant).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Tomita (first author).
Conflict of interest
There is no conflict of interest to disclose.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board of the University of the Ryukyus.
Ethical approval
Institutional Review Board approval was obtained at the University of the Ryukyus.
Methodology
• Retrospective
• Observational study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 15.6 kb)
Appendix 1. The list of Texture features
Appendix 1. The list of Texture features
Histogram features
Histogram features describe simple statistics that are associated with pixel values in images. Spatial patterns of pixel values are not included. Skewness, kurtosis, entropy, and energy were calculated.
Gray-level co-occurrence matrix (GLCM) features
The GLCM is defined as the distribution of co-occurring pixel values that are calculated from 4 directions in 2-dimensional (2D) space or 13 directions in 3-dimensional (3D) space:
Where p(i,j) represents (i,j) value of the GLCM.
Gray-level run length matrix (GLRLM) features
The GLRLM is defined as the number of consecutive pixels of the same gray-level value for 4 directions in 2D space or 13 directions in 3D space:
Short run emphasis \( \left(\mathrm{SRE}\right)=\frac{1}{n_r}\sum \limits_{i,j}\frac{p\left(i,j\right)}{j^2} \)
Low gray-level run emphasis \( \left(\mathrm{LGRE}\right)=\frac{1}{n_r}\sum \limits_{i,j}\frac{p\left(i,j\right)}{i^2} \)
High gray-level run emphasis \( \left(\mathrm{HGRE}\right)=\frac{1}{n_r}\sum \limits_{i,j}p\left(i,j\right){i}^2 \)
Short run low gray-level emphasis \( \left(\mathrm{SRLGE}\right)=\frac{1}{n_r}\sum \limits_{i,j}\frac{p\left(i,j\right)}{i^2{j}^2} \)
Short run high gray-level emphasis \( \left(\mathrm{SRHGE}\right)=\frac{1}{n_r}\sum \limits_{i,j}\frac{p\left(i,j\right){i}^2}{j^2} \)
Run percentage \( \left(\mathrm{RP}\right)=\frac{n_r}{\sum \limits_{i,j}\left( jp\left(i,j\right)\right)} \)Where nr corresponds to the number of homogenous runs.
Neighborhood gray-level different matrix (NGLDM)
The NGLDM is defined as the difference in gray-levels between adjacent voxels of 8 in 2D space and 26 in 3D space:
Where E is the number of voxels in VOI and G is the number of gray-levels.
Gray-level zone length matrix (GLZLM)
GLZLM is defined as the number of homogenous zones of the same gray-level value in 2D or 3D space:
Short-zone emphasis \( \left(\mathrm{SZE}\right)=\frac{1}{n_r}\sum \limits_{i,j}\frac{p\left(i,j\right)}{j^2} \)
Low gray-level zone emphasis \( \left(\mathrm{LGZE}\right)=\frac{1}{n_r}\sum \limits_{i,j}\frac{p\left(i,j\right)}{i^2} \)
High gray-level zone emphasis \( \left(\mathrm{HGZE}\right)=\frac{1}{n_r}\sum \limits_{i,j}p\left(i,j\right){i}^2 \)
Short-zone low gray-level emphasis \( \left(\mathrm{SZLGE}\right)=\frac{1}{n_r}\sum \limits_{i,j}\frac{p\left(i,j\right)}{i^2{j}^2} \)
Short-zone high gray-level emphasis \( \left(\mathrm{SZHGE}\right)=\frac{1}{n_r}\sum \limits_{i,j}\frac{p\left(i,j\right){i}^2}{j^2} \)
Zone length non-uniformity \( \left(\mathrm{ZLNU}\right)=\frac{1}{n_r}\sum \limits_j{\left(\sum \limits_ip\left(i,j\right)\right)}^2 \)
Zone percentage \( \left(\mathrm{ZP}\right)=\frac{n_r}{\sum \limits_{i,j}\left( jp\left(i,j\right)\right)} \)where nr corresponds to the number of homogenous zones.
Rights and permissions
About this article
Cite this article
Tomita, H., Yamashiro, T., Heianna, J. et al. Nodal-based radiomics analysis for identifying cervical lymph node metastasis at levels I and II in patients with oral squamous cell carcinoma using contrast-enhanced computed tomography. Eur Radiol 31, 7440–7449 (2021). https://doi.org/10.1007/s00330-021-07758-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07758-4