Abstract
Objectives
To survey (1) operator’s eye lens doses in typical computed tomography (CT)-guided interventions, (2) correlation between dose length product (DLP) and the operator’s dose, and (3) different ways for estimating the eye lens dose in clinical settings.
Methods
Doses of 16 radiologists in 164 CT-guided interventional procedures were prospectively measured during a 6-month time period upon radioprotective garments and descriptive statistical outcomes were calculated. The correlations between DLP and measured doses were surveyed.
Results
On average, the operator’s dose at the eye level (DEL, Hp(0.07)) was 22 μSv per procedure and the personal equivalent dose Hp(10) at the collar level was 21 μSv per procedure. The mean DLP of a procedure was 320 mGy cm, where 54% resulted from the fluoroscopy, the mean exposure time being 18 s. Based on the results, the operator’s DEL could be estimated from DLP using the equation DEL (μSv) = 0.10 μSv/mGy cm × patient fluoro DLP (mGycm) (p < 0.001), and the dose at the collar level (DCL) using the equation DCL (μSv) = 0.12 μSv/mGy cm × patient fluoro DLP (mGy cm) (p < 0.001). In addition, DEL (μSv) = 0.7 × DCL (μSv).
Conclusions
The eye lens doses in CT-guided interventions are generally low even without protective equipment, and it is unlikely that the recommended annual equivalent dose limit of 20 mSv for the lens of the eye will be exceeded by conducting CT-guided interventions solely. Eye lens dose can be roughly estimated based on either DLP of the procedure or dose measured at the operator’s collar level.
Key Points
• Eye lens doses in CT-guided operations are generally low.
• It is unlikely that the ICRP recommendation of the yearly equivalent dose limit of 20 mSv will be exceeded by conducting CT-guided interventions solely.
• Magnitude of eye lens dose can be estimated based on either DLP of the procedure or dose measured at the operator’s collar level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Operators are exposed to variable levels of ionizing radiation at their work due to X-ray-guided patient interventions and examinations. It has been recognized that ionizing radiation may cause cataract: ocular lens opacity with visual impairment [1]. Previously, lens opacities were believed to occur after acute exposures to 0.5–2 Sv or more and vision-impairing cataracts after 5 Sv [1,2,3]. However, recent data from animal models and human populations suggest that the limit for the potentially harmful dose is considerably lower and could result in e.g. changes in contrast sensitivity, blurred vision, and loss of visual acuity [4,5,6,7,8,9,10,11]. Consequently, the International Commission on Radiological Protection (ICRP) published a recommendation in 2012 for a new dose threshold for visual-impairing cataracts of 0.5 Gy and lowered the recommendation for occupational dose limit for eye lens from 150 to 20 mSv per year [12]. Since then, the occupational exposure of the eye has gained attention related to e.g. cardiological procedures [13,14,15,16,17], hybrid operating rooms [18], and neuroradiological procedures [19]. However, until now, the eye doses in computed tomography (CT)-guided interventions have not been well documented regardless of routine use. In the literature review, only one paper [20] studying eye lens doses of the staff in CT-guided interventions was found. Thus, more knowledge about typical occupational eye doses from CT-guided interventions and about optimal ways to determine them are needed.
Typical CT-guided interventions are biopsies, drainages, and hyperthermal tumor therapies [21] in which a traditional CT scan is firstly used for planning the procedure. During the intervention, real-time fluoroscopic CT is used to provide the operator’s immediate feedback, i.e., for targeting a biopsy needle. In most procedures, the operator has to stay close to the patient while using fluoroscopy. In some procedures, it is possible to use protective extra shields like a lead glass shield, but in many cases only personal shielding like aprons and glasses can be employed. The durations of the procedures can vary extensively from minutes to hours where the fluoroscopic exposure time varies from seconds to minutes. Thus, in some cases, the operator’s radiation exposure can be notable.
In clinical radiology, different kinds of metrics and dosimeters are used to estimate and monitor radiation doses of personnel [21]. Equivalent dose HT (mSv) is a measure of radiation dose to an organ or tissue (T) which also takes into account the quality of the radiation. The personal (P) dose equivalents Hp(10) and Hp(0.07) (mSv) are measures of radiation doses at 10 mm and 0.07 mm depth, respectively, from skin surface at the measurement site. Hp(10) reflects the personal effective dose and is also called the deep dose equivalent and Hp(0.07) is used as equivalent to the skin dose. To monitor the dose to the eye lens, measurement of Hp(3) is recommended by ICRP. However, for now, very few physical dosimeters are available and/or calibrated to actually measure Hp(3). For example in Finland, there is currently no certified dosimeter available for measuring the eye lens doses.
The purpose of this study was to survey (1) operator’s eye lens doses in typical CT-guided interventions, (2) correlation between DLP and operator’s dose, and (3) different ways for estimating the eye lens dose in clinical settings.
Materials and methods
This prospective single-center study, in which the eye lens doses of operators were measured with multiple dosimeters during various CT-guided interventions, was approved by the institutional review board of Kuopio University Hospital. The study was conducted at the Department of Clinical Radiology in Kuopio University Hospital (Kuopio, Finland) where around 300 CT-guided operations are yearly conducted.
In this prospective study, dose measurements of operators were conducted in 164 CT-guided interventional procedures during a 6-month time period. The procedures included 59 epidural steroid injections, 57 lung biopsies, 16 bone biopsies, 16 drainages, eight pre-operative tumor marker placements, five soft tissue biopsies, and three tumor ablations. All 16 radiologists that performed CT-guided procedures were included in the study (12 male, 4 female). On average, the operators had 7 years of experience in CT-guided operations (range 1–20 years, median 6 years). The height of the operators was 177 cm in average (range 165–187 cm, median 178 cm). The operators wore thyroid shields and lead aprons in all procedures, and lead glasses in the majority of the procedures (70%). Only when the radiologist was able to stay behind the lead glass shield were radiation protective glasses not worn. The dosimeters were placed so that the personal protective garments did not cover them. The lead equivalence of the thyroid shield, lead apron, and lead glasses was 0.5 mm at 100 kV.
The procedures were performed either on Somatom Edge (Siemens AG, 64 detector rows, 78-cm gantry opening) or Sensation 16 (Siemens AG, 16 detector rows, 70-cm gantry opening) multi-detector CTs. First, a planning CT scan was performed during which the personnel was not in the CT room. Based on the planning CT scan, an optimal route and accessories for the procedure were determined. Then, the operator, staying in the CT room, used CT fluoroscopy to guide the procedure (100–120 kV, 30–80 mAs, collimation 6 × 1.2 mm). The height of the patient table was 152 cm on average (range 126–172, median 156 cm). The operator’s distance from the patient and fluoro scan area varied depending on the type of the operation: In the majority of the procedures, the operator needed to hold the instruments and stay approximately 30–50 cm from the fluoroscopy area (Fig. 1a). In some of the procedures (most epidural steroid injections and some drainages and tumor biopsies), the operators were able to stand behind the lead wall (180 × 90 cm, 0.5 mm Pb at 100 kV) during fluoroscopy without having to constantly hold the instruments (Fig. 1b). After each procedure, the operator gave a subjective evaluation of the complexity of the procedure on a scale of 1–3 (1 easy, 2 moderate, 3 difficult).
A typical positioning of the operators during a CT guided operation where (a) the operator needed to hold the instruments and stay approximately 30–50 cm from the fluoroscopy area and (b) the operators were able to stand behind the lead wall during fluoroscopy without having to constantly hold the instruments. The operator is on the left and the assisting operator on the right. In this particular procedure, the operator is not wearing lead glasses, the assistant operator is
The eye lens doses were measured using a prototype of an eye dosimetry headband using a Harshaw ExtradTM dosimeter element which was calibrated for Hp(3) measurements (Rotunda Scientific Technologies). Furthermore, as there was no certified method for eye lens dose measurements Hp(3), routine dosimeters (measuring Hp(0.07) and Hp(10)) were also used in order to study the relationship between the doses measured with routinely used dosimeters and the eye lens dosimeter.
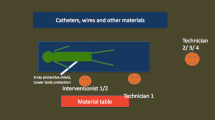
During each procedure, the operator wore two dosimeters at eye level and two dosimeters at collar level (Fig. 2). The radiation doses were measured so that the protective garments did not cover the dosimeters. At the collar level, a regular passive personal thermo-luminescent detector (TLD, Doseco) was attached to the thyroid shield and used for measuring the cumulative personal doses (Hp(10) and Hp(0.07)). In order to measure the personal equivalent dose per procedure at the collar level, also an active RAD-60 personal electronic dosimeter (Mirion Technologies) was used. The eye lens dose per procedure was measured with an Educational Direct Dosimeter 30 (EDD-30, Unfors Instruments) and the sensor was placed on the forehead above the eyes. The EDD-30 was calibrated in terms of Hp(0.07). Furthermore, a prototype headband (Rotunda Scientific Technologies) with a Harshaw ExtradTM dosimeter element was used for measuring the cumulative eye lens dose for eight radiologists. The product incorporated a 300 mg/cm2 polytetrafluoroetylene filter and was calibrated for measuring Hp(3). The headband was worn so that a middle TLD laid between the eyes on the forehead. The headband (with limited availability) was used only for the radiologists who conduct the majority of the procedures at the department. The number of different procedures performed by radiologists wearing Rotunda headband is given in Table 3. The calibration accuracies, energy responses, and angular dependencies of the dosimeters are given in Table 1.
Placement of the dosimeters. Dose was measured with (1) a TLD attached to a headband at the middle of the forehead (Rotunda headband) (Hp(3)) and with (2) EDD-30 placed on the forehead of the radiologist (Hp(0.07)). Total dose was measured with (3) a regular TLD attached to the thyroid shield (Hp(10)) and with (4) RAD-60 personal electronic dosimeter at collar level (Hp(10))
The passive dosimeters were read in a company (Doseco Oy) that is a certified monitoring service company approved by the Finnish Radiation and Nuclear Safety Authority (STUK). The readout of the passive dosimeters (Rotunda headband with a Harshaw ExtradTM dosemeter element and TLD dosimeters held above the thyroid shield) was done every month or every three months depending on the operator using the dosimeter: some of the operators are class A radiation workers whose dosimeters are read monthly and some operators are class B radiation workers whose dosimeters are read every three months. The background subtraction for the passive dosimeters was conducted at the same time. The readout of the active dosimeters (RAD-60 and EDD-30) was done after every procedure and no background subtraction was conducted.
The statistical analyses were done using SPSS 23.0.0.2 software (IBM). A power analysis was used for determining an adequate sample size. Quantitative parameters are expressed as mean values and ranges. Student’s t test was used for comparing the statistical differences between the groups. The Spearman correlation coefficient, with its corresponding p value, was used to examine the relationship between operator’s lens doses and the doses measured with various dosimeters, the fluoroscopy time, and the patient fluoroscopy DLP. Scatter plots with a regression line were used to illustrate correlations between quantitative parameters.
Results
Operator’s doses in various procedures
In procedures where the operator stayed behind the lead screen (n = 47), the mean dose per procedure was below the detection level of the dosimeters. These measurements were not included in the further description and analysis of results. The DLP was smallest in epidural steroid injections (183 mGy cm, range 19–527 mGy cm, median 154 mGy cm).
On average, the dose of the operator (working without a lead wall) at the eye level (Hp(0.07)) was 22 μSv per procedure (range 0.04–89 μSv, median 15 μSv) (Table 2). The average personal equivalent dose Hp(10) at the collar level was 21 μSv per procedure (range 1–128 μSv, median 12 μSv). The mean DLP of a patient was 320 mGy cm (range 57–1210 mGy cm, median 250 mGy cm), where 54% (174 mGy cm, range 14–962 mGy cm, median 115 mGy cm) resulted from the fluoroscopy with an average exposure time 18 s (range 13–107 s, median 13 s). The largest doses at the eye level resulted from tumor ablations, where the average dose (Hp(0.07)) was 75 μSv (range 49–89 μSv). The detailed results are presented in Tables 2 and 3. Eighty-five procedures were performed from the right side of the CT gantry and 79 procedures from the left side. There was no difference in the measured doses Hp(0.07 and 10) of the radiologist or the patient depending on the side (p > 0.1). The average estimated complexity of the procedures was 1.5 and there was no difference in the estimated complexity between the two devices used. The results are presented for both the devices together.
Measuring and estimating the operator’s dose in CT-guided procedures
Measurements with active dosimeters
There was a statistically significant correlation between the fluoroscopy DLP (mGy cm) and the dose of the operator measured at the eye level with the EDD-30 (Hp(0.07), μSv) (correlation coefficient r = 0.78, r2 = 0.60, p < 0.001, n = 106, confidence interval S (95%) = 29, standard error of estimate 12 μSv, DEL/DLP range 0.0003–0.6; Fig. 3). Based on our results, the dose (Hp(0.07)) at eye level (DEL) can be estimated from the DLP using the following Eq. 1:
The personal dose of the radiologist measured at collar level (DCL) with RAD-60 Hp(10) also correlated significantly with the DLP from fluoroscopy mode (r = 0.81, r2 = 0.66, p < 0.001, S (95%) = 24.6, n = 106, standard error of estimate 14 μSv, DCL/DLP range 0.01–0.7; Fig. 3). Thus, the personnel dose (Hp(10)) at collar level can be estimated using the following Eq. 2:
There was a statistically significant correlation between the doses measured with EDD (Hp(0.07) μSv) at eye level and RAD-60 (Hp(10), μSv) at collar level (r = 0.87, r2 = 0.76, p < 0.001, S (95%) = 22.8, n = 106, standard error of estimate 10 μSv, DEL/DCL range 0.04–4.8; Fig. 4) and the dose at eye level (DEL, Hp(0.07)) can be estimated using Eq. 3:
Measurements with passive dosimeters
There was no statistically significant correlation between the measurements with Rotunda headband (middle dosimeter) and EDD-30 (Table 4, p = 0.889). There was no statistically significant correlation between the TLD at collar level and RAD-60 (Table 4, p = 0.123).
Discussion
The average dose at eye level Hp(0.07) in a total of 164 CT-guided interventions was 22 μSv. Thus, it can be estimated that around 900 yearly procedures would be needed in order to exceed the ICRP recommendation of the annual eye lens dose limit of 20 mSv. This would require more than 17 CT-guided procedures in a week, per person. The largest doses at eye level resulted from tumor ablations (Hp(0.07), 75 μSv on average) and the smallest in epidural steroid injections when the operator stayed behind a lead screen further from the patient (Hp(0.07), result below the detection level of the dosimeters). Hence, it is generally safe to say that it is unlikely to exceed the annual eye lens dose recommendation by performing CT-guided operations only. All of the doses were measured upon protective garments, so the real eye lens dose when using e.g. lead glasses is even smaller.
On the other hand, the highest dose at eye level Hp(0.07) was 89 μSv during a tumor ablation and around 4 similar procedures weekly would suffice to exceed the annual limit. This highlights the importance of (a) measuring personnel eye lens doses and (b) use of lead glasses also in CT-guided procedures, similar to other high-dose modalities.
Our results demonstrate that the operator’s dose at eye level and dose at collar level in CT-guided interventions can be roughly estimated from the patient dose-relevant parameter DLP using specific formulae. The placement of the dosimeter at collar level was based on a recommendation given by ICRP [23] and the placement of the dosimeter above the lead shield was based on instructions given by Finnish Radiation and Nuclear Safety Authority. It should be noted that the backscattered component is eliminated by the lead shield and is not included in the result. The placement of the dosimeter on the forehead of the operator was such that it was not directly covered by the lead glasses used by the operator. The formula to estimate the dose based on patient dose-relevant parameter DLP was almost the same for DEL and DCL. This highlights the fact that the accuracy of the estimation is better with actually measuring the dose at collar level compared to only relying on DLP data. The accuracy and robustness of this suggested method are a scope of more extensive studies. However, the formulae given in this paper may provide good general estimates for many practical purposes.
Our results showed a statistically significant correlation between the dose measured with EDD-30 at eye level and dose measured with RAD-60 at collar level. Our result is of similar magnitude as in a simulation study where eye dose was found to be 0.75× neck dose in interventional procedures [24]. This further highlights that although different dosimeters have different properties in terms of calibration, energy, and angular dependency, it is still possible to use commonly existing dosimeters for estimating the level of occupational eye lens dose.
The tumor ablations were rated more difficultly than average tumor ablations (2.3). The epidural steroid injections were rated to be easier than normal (1.2). These operations also gave the highest and lowest DLPs, respectively. In the future, the subjective scaling could be improved with quantitative complexity indices.
Eye lens doses in CT-guided interventions have been previously studied by Heusch et al [20]. In their study, the median total exposure of the eye lens was 3.3 μSv. In our study, the measured eye lens doses were higher which might be due to the differences in measurement site, fluoroscopic parameters, workflow, patient size, and difficulty of the procedures. Nevertheless, both studies demonstrate that performing only CT-guided operations, the yearly eye lens dose recommendation is unlikely exceeded.
There are a few limitations in our study. First, DEL was measured with Rotunda headband in 117 procedures altogether. However, only three out of eight radiologists performed procedures that resulted in substantial DELs which limited the feasibility of statistical methods on that data. There was, however, a relationship between the measurements with DEL and DCL which shows that the linkage should be studied in more detail with future measurements. Despite the difficulty comparing the groups, there can been seen a difference in the dose levels: the doses measured with TLD were higher compared to the doses measured with RAD-60. The operations were conducted with energies up to 120 kV, where the energy of the scattered photons is in the range of around 20–110 keV (mean 52 keV) [25]. There is a difference in the energy response of RAD-60 and TLD (see Table 1). As TLD is able to measure doses at lower energies, this could explain the higher doses measured with the TLD. In addition, the doses measured with the headband were higher compared to the EDD measurements. The energy dependence for EDD is less than 25% in the energy range of 20–65 kV. As the measurable energy range for the head band is wider (less than 20%, 20–662 keV), this might explain the results. In the future, more data points for passive dosimeters are needed to confirm the results. Secondly, numbers of some procedure types were small (n < 10). Both of these issues should be taken into account in future study designs.
The distance of the operator from the gantry and patient also affects the personnel doses notably as one of the factors affecting the radiation dose is the distance of the object from the source/scattering source. Thus, also the height of the operator and height of the patient table affect the result. As we concentrated on estimating the radiation dose in clinical settings, we have not evaluated this matter in our research in detail and this could be addressed in the future. In this practical study, the distances and operators’ movements were not controlled and were described only with limited accuracy. However, we believe that the practices in procedures are somewhat generic: the operator either stays next to the moving target or is able to stand further away behind a lead screen. Both of these cases have been taken into account in this study.
One potential limitation of this study is that it is based on data from a single center. This might diminish the generalizability of the results. In the future, it would be beneficial to gather data from other centers too.
In conclusion, eye lens doses in CT-guided operations are generally low and it is unlikely that the recommended annual equivalent dose limit of 20 mSv for the lens of the eye will be exceeded solely by conducting CT-guided interventions even without protective eyewear. Dose at eye level can be estimated using a formula based on either fluoro DLP or dose measured at collar level.
Abbreviations
- CT:
-
Computed tomography
- DCL:
-
Dose at collar level
- DEL:
-
Dose at eye level
- DLP:
-
Dose length product
- EDD:
-
Educational Direct Dosimeter
- Hp(0.07):
-
Dose equivalent at the depth of 0.07 mm
- Hp(10):
-
Dose equivalent at the depth of 10 mm
- Hp(3):
-
Dose equivalent at the depth of 3 mm
- ICRP:
-
International Commission on Radiological Protection
- TLDs:
-
Thermo-luminescent detector
References
Shore RE, Neriishi K, Nakashima E (2010) Epidemiological studies of cataract risk at low to moderate radiation doses: (not) seeing is believing. Radiat Res 174(6):889–894
Hammer GP, Scheidemann-Wesp U, Samkange-Zeeb F, Wicke H, Neriishi K, Blettner M (2013) Occupational exposure to low doses of ionizing radiation and cataract development: a systematic literature review and perspectives on future studies. Radiat Environ Biophys 52(3):303–319
ICRP (1991) Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP 21 (1–3)
Chodick G, Bekiroglu N, Hauptmann M et al (2008) Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol 168(6):620–631
Klein BE, Klein R, Linton KL et al (1993) Diagnostic X ray exposure and lens opacities: the Beaver Dam Eye Study. Am J Public Health 83:588–590
Wilde G, Sjöstrand J (1997) A clinical study of radiation cataract formation in adult life following γ irradiation of the lens in early childhood. Br J Ophthalmol 81(4):261–266
Hall P, Granath F, Lundell M, Olsson K, Holm LE (1999) Lenticular opacities in individuals exposed to ionizing radiation in infancy. Radiat Res 152(2):190–195
Cucinotta FA, Kim MH, Willingham V (2008) Physical and biological organ dosimetry analysis for international space station astronauts. Radiat Res 170:127–138
Rastegar N, Eckart P, Mertz M (2002) Radiation-induced cataract in astronauts and cosmonauts. Graefes Arch Clin Exp Ophthalmol 240:543–547
Junk AK, Haskal Z, Worgul BV (2004) Cataract in interventional radiology — an occupational hazard? Invest Ophthalmol Vis Sci 45(Suppl. S2005):388
Kleiman NJ, Cabrera M, Duran G, Ramirez R, Duran A, Vañó, E (2009) Occupational risk of radiation cataract in interventional cardiology. Invest Ophthalmol Vis Sci 49, Presentation abstract 511/D656
ICRP (2012) ICRP statement on tissue reactions / early and late effects of radiation in normal tissues and organs – threshold doses for tissue reactions in a radiation protection context. ICRP Publication 118. Ann ICRP 41(1/2)
Vanhavere F, Carinou E, Domienik J et al (2011) Measurements of eye lens doses in interventional radiology and cardiology: final results of the ORAMED project. Radiat Meas 46:1243–1247
Donadille L, Carinou E, Brodecki M et al (2011) Staff eye lens and extremity exposure in interventional cardiology: results of the ORAMED project. Radiat Meas 46:1203–1209
Carinou E, Brodecki M, Domienik J et al (2011) Recommendations to reduce extremity and eye lens doses in interventional radiology and cardiology. Radiat Meas 46:1324–1329
Krim S, Brodecki M, Carinou E et al (2011) Extremity doses of medical staff involved in interventional radiology and cardiology: correlations and annual doses (hands and legs). Radiat Meas 46:1223–1227
Omar A, Kadesjö N, Palmgren C, Marteinsdottir M, Segerdahl T, Fransson A (2017) Assessment of the occupational eye lens dose for clinical staff in interventional radiology, cardiology and neuroradiology. J Radiol Prot 37(1):145–159
Attigah N, Oikonomou K, Hinz U et al (2016) Radiation exposure to eye lens and operator hands during endovascular procedures in hybrid operating rooms. J Vasc Surg 63(1):198–203
Sans Merce M, Korchi AM, Kobzeva L et al (2016) The value of protective head cap and glasses in neurointerventional radiology. J Neurointervent Surg 8:736–740
Philipp Heusch P, Kröpil P, Buchbender C et al (2014) Radiation exposure of the radiologist’s eye lens during CT-guided interventions. Acta Radiol 55(1):86–90
Begemann P (2009) CT-Guided interventions – indications, technique, pitfalls. In: Mahnken A, Ricke J (eds) CT- and MR-guided interventions in radiology. Springer, Berlin Heidelberg
Gilvin P, Baker S, Gibbens N et al (2013) Type testing of a head band dosemeter for measuring eye lens dose in terms of Hp(3). Radiat Prot Dosimetry 157(3):430–436
ICRP (2013) Radiological protection in cardiology. ICRP Publication 120. Ann ICRP 42(1)
Martin C (2011) Personal dosimetry for interventional operators: when and how should monitoring be done? Br J Radiol 84:639–648
Platten D (2014) A Monte Carlo study of the energy spectra and transmission characteristics of scattered radiation from X-ray computed tomography. J Radiol Prot 34(2)
Acknowledgments
We thank Tuomas Selander for support in statistical analysis
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hanna Matikka, PhD, Kuopio University Hospital.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Tuomas Selander kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• experimental
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaartinen, S., Husso, M. & Matikka, H. Operator’s eye lens dose in computed tomography–guided interventions. Eur Radiol 31, 4377–4385 (2021). https://doi.org/10.1007/s00330-020-07576-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07576-0