Abstract
Objective
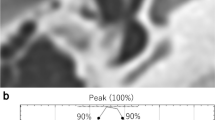
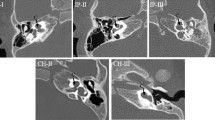
Diagnosis of otosclerosis on temporal bone CT images is often difficult because the imaging findings are frequently subtle. Our aim was to assess the utility of deep learning analysis in diagnosing otosclerosis on temporal bone CT images.
Methods
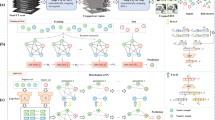
A total of 198 temporal bone CT images were divided into the training set (n = 140) and the test set (n = 58). The final diagnosis (otosclerosis-positive or otosclerosis-negative) was determined by an experienced senior radiologist who carefully reviewed all 198 temporal bone CT images while correlating with clinical and intraoperative findings. In deep learning analysis, a rectangular target region that includes the area of the fissula ante fenestram was extracted and fed into the deep learning training sessions to create a diagnostic model. Transfer learning was used with the deep learning model architectures of AlexNet, VGGNet, GoogLeNet, and ResNet. The test data set was subsequently analyzed using these models and by another radiologist with 3 years of experience in neuroradiology following completion of a neuroradiology fellowship. The performance of the radiologist and the deep learning models was determined using the senior radiologist’s diagnosis as the gold standard.
Results
The diagnostic accuracies were 0.89, 0.72, 0.81, 0.86, and 0.86 for the subspecialty trained radiologist, AlexNet, VGGNet, GoogLeNet, and ResNet, respectively. The performances of VGGNet, GoogLeNet, and ResNet were not significantly different compared to the radiologist. In addition, GoogLeNet and ResNet demonstrated non-inferiority compared to the radiologist.
Conclusions
Deep learning technique may be a useful supportive tool in diagnosing otosclerosis on temporal bone CT.
Key Points
• Deep learning can be a helpful tool for the diagnosis of otosclerosis on temporal bone CT.
• Deep learning analyses with GoogLeNet and ResNet demonstrate non-inferiority when compared to the subspecialty trained radiologist.
• Deep learning may be particularly useful in medical institutions without experienced radiologists.


Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- DICOM:
-
Digital Imaging and Communications in Medicine
- HU:
-
Hounsfield unit
- JPEG:
-
Joint Photographic Experts Group
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristics
- ROI:
-
Regions of interest
References
Davis GL (1987) Pathology of otosclerosis: a review. Am J Otolaryngol 8:273–281. https://doi.org/10.1016/s0196-0709(87)80046-7
Cureoglu S, Schachern PA, Ferlito A, Rinaldo A, Tsuprun V, Paparella MM (2006) Otosclerosis: etiopathogenesis and histopathology. Am J Otolaryngol 27:334–340. https://doi.org/10.1016/j.amjoto.2005.11.001
Quesnel AM, Ishai R, McKenna MJ (2018) Otosclerosis: temporal bone pathology. Otolaryngol Clin North Am 51:291–303. https://doi.org/10.1016/j.otc.2017.11.001
Sakai O, Curtin HD, Hasso AN, Swartz JD (2011) Otosclerosis and dysplasias of the temporal bone. In: Som PM, Curtin HD (eds) Head and neck imaging, 5th edn. Elsevier Mosby, Philadelphia, pp 1231–1261
Andreu-Arasa VC, Sung EK, Fujita A, Saito N, Sakai O (2019) Otosclerosis and dysplasias of the temporal bone. Neuroimaging Clin N Am 29:29–47. https://doi.org/10.1016/j.nic.2018.09.004
Purohit B, Hermans R, Op de Beeck K (2014) Imaging in otosclerosis: a pictorial review. Insights Imaging 5:245–252. https://doi.org/10.1007/s13244-014-0313-9
Chole RA, McKenna M (2001) Pathophysiology of otosclerosis. Otol Neurotol 22:249–257. https://doi.org/10.1097/00129492-200103000-00023
Lagleyre S, Sorrentino T, Calmels MN et al (2009) Reliability of high-resolution CT scan in diagnosis of otosclerosis. Otol Neurotol 30:1152–1159. https://doi.org/10.1097/MAO.0b013e3181c2a084
Kanzara T, Virk JS (2017) Diagnostic performance of high resolution computed tomography in otosclerosis. World J Clin Cases 5:286–291. https://doi.org/10.12998/wjcc.v5.i7.286
Dreyer KJ, Geis JR (2017) When machines think: radiology’s next Frontier. Radiology 285:713–718. https://doi.org/10.1148/radiol.2017171183
Soffer S, Ben-Cohen A, Shimon O, Amitai MM, Greenspan H, Klang E (2019) Convolutional neural networks for radiologic images: a radiologist’s guide. Radiology 290:590–606. https://doi.org/10.1148/radiol.2018180547
Rodriguez-Ruiz A, Krupinski E, Mordang JJ et al (2019) Detection of breast cancer with mammography: effect of an artificial intelligence support system. Radiology 290:305–314. https://doi.org/10.1148/radiol.2018181371
McKinney SM, Sieniek M, Godbole V et al (2020) International evaluation of an AI system for breast cancer screening. Nature 577:89–94. https://doi.org/10.1038/s41586-019-1799-6
Krizhevsky A, Sutskever I, Hinton G (2012) ImageNet classification with deep convolutional neural networks. Proc Adv Neural Inf Process Syst 1097–1105. https://doi.org/10.1145/3065386
Simonyan K, Zisserman A (2014) Very deep convolutional networks for large-scale image recognition. arXiv arXiv:1409. https://arxiv.org/abs/1409.1556. Accessed 11 Oct 2019
Szegedy C, Liu W, Jia Y et al (2015) Going deeper with convolutions. Proc IEEE Conf Comput Vis Pattern Recognit 1–9. https://doi.org/10.1109/CVPR.2015.7298594
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. Proc IEEE Conf Comput Vis Pattern Recognit 770–778. https://doi.org/10.1109/CVPR.2016.90
Thomas JL, Ridner M, Cole JH et al (2018) The clinical evaluation of the CADence device in the acoustic detection of coronary artery disease. Int J Cardiovasc Imaging 34:1841–1848. https://doi.org/10.1007/s10554-018-1403-4
Grayeli AB, Yrieix CS, Imauchi Y, Cyna-Gorse F, Ferrary E, Sterkers O (2004) Temporal bone density measurements using CT in otosclerosis. Acta Otolaryngol 124:1136–1140. https://doi.org/10.1080/00016480410018188
Kawase S, Naganawa S, Sone M, Ikeda M, Ishigaki T (2006) Relationship between CT densitometry with a slice thickness of 0.5 mm and audiometry in otosclerosis. Eur Radiol 16:1367–1373. https://doi.org/10.1007/s00330-005-0128-7
Yamashita K, Yoshiura T, Hiwatashi A et al (2014) The radiological diagnosis of fenestral otosclerosis: the utility of histogram analysis using multidetector row CT. Eur Arch Otorhinolaryngol 271:3277–3282. https://doi.org/10.1007/s00405-014-2933-6
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Osamu Sakai.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
All study cohorts have not been previously reported in scientific papers. However, images of 5 patients out of 99 patients were presented in a review article: Andreu-Arasa VC, Sung EK, Fujita A, Saito N, Sakai O. Otosclerosis and Dysplasias of the Temporal Bone. Neuroimaging Clin N Am. 2019;29(1):29-47. doi:10.1016/j.nic.2018.09.004.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujima, N., Andreu-Arasa, V.C., Onoue, K. et al. Utility of deep learning for the diagnosis of otosclerosis on temporal bone CT. Eur Radiol 31, 5206–5211 (2021). https://doi.org/10.1007/s00330-020-07568-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07568-0