Abstract
Objectives
To evaluate the efficiency of 2- and 3-class classification predictive tasks constructed from radiomics features extracted from dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) pharmacokinetic (PK) protocol in discriminating among benign, borderline, and malignant ovarian tumors.
Methods
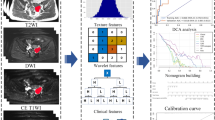
One hundred and four ovarian lesions were evaluated using preoperative DCE-MRI. Radiomics features were extracted from 7 types of DCE-MR images. To explore the differential ability of radiomics between three types of ovarian tumors, two- and three-class classification tasks were established. The 2-class classification task was divided into three subtasks: benign vs. borderline (task A), benign vs. malignant (task B), and borderline vs. malignant (task C). For the 3-class classification task, 104 lesions were randomly divided into training (72 lesions) and validation (32 lesions) cohorts. The discrimination abilities of the radiomics signatures were established with the training cohort and tested with the independent validation cohort. The predictive performance of the task was evaluated by receiver operating characteristic (ROC) curve, calibration curve analysis, and decision curve analysis (DCA).
Results
For the 2-class classification task, the combination of PK radiomics signatures model (PK model) showed a good diagnostic ability with the highest area under the ROC curves (AUCs) of 0.899, 0.865, and 0.893 for tasks A, B, and C, respectively. Additionally, the 3-class classification task demonstrated a good discrimination performance with AUCs of 0.893, 0.944, and 0.891 for the benign, borderline, and malignant groups, respectively.
Conclusions
Radiomics analysis based on the DCE-MRI PK protocol showed promise for discriminating among benign, borderline, and malignant ovarian tumors.
Key Points
• Two-class classification predictive task of DCE-MRI PK protocol enabled the classification of 3 categories of ovarian tumors through the pairwise comparison strategy with a perfect diagnostic ability.
• Three-class classification predictive task maintained good performance to effectively judge each category of ovarian tumors directly.




Similar content being viewed by others
Abbreviations
- AIF:
-
Arterial input function
- AUC:
-
Area under the ROI curve
- CER:
-
Contrast-enhanced ratio
- DCA:
-
Decision curve analysis
- DCE:
-
Dynamic contrast enhanced
- fPV:
-
Blood plasma volume
- IAUGC:
-
Initial area under the gadolinium contrast agent concentration time curve
- Kep :
-
Rate of contrast agent transport from the tumor to the blood
- Ktrans :
-
Rate of contrast agent uptake into the tumor from the blood
- LAVA:
-
Liver acquisition with volume acceleration
- MRI:
-
Magnetic resonance imaging
- mRMR:
-
Minimum redundancy maximum relevance
- ROC:
-
Receiver operating characteristic
- Ve :
-
Volume of the extravascular extracellular space
- VOI:
-
Volume of interest
References
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA (2014) Ovarian cancer. Lancet 384:1376–1388
Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC (2001) Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 22:255–288
Fischerova D, Zikan M, Dundr P, Cibula D (2012) Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist 17:1515–1533
Spencer JA, Ghattamaneni S (2010) MR imaging of the sonographically indeterminate adnexal mass. Radiology 256:677–694
Adusumilli S, Hussain HK, Caoili EM et al (2006) MRI of sonographically indeterminate adnexal masses. AJR Am J Roentgenol 187:732–740
Jeong YY, Outwater EK, Kang HK (2000) Imaging evaluation of ovarian masses. Radiographics 20:1445–1470
Thomassin-Naggara I, Soualhi N, Balvay D, Darai E, Cuenod CA (2017) Quantifying tumor vascular heterogeneity with DCE-MRI in complex adnexal masses: a preliminary study. J Magn Reson Imaging 46:1776–1785
Thomassin-Naggara I, Darai E, Cuenod CA, Rouzier R, Callard P, Bazot M (2008) Dynamic contrast-enhanced magnetic resonance imaging: a useful tool for characterizing ovarian epithelial tumors. J Magn Reson Imaging 28:111–120
Li HM, Qiang JW, Ma FH, Zhao SH (2017) The value of dynamic contrast-enhanced MRI in characterizing complex ovarian tumors. J Ovarian Res 10:4
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Rizzo S, Botta F, Raimondi S et al (2018) Radiomics of high-grade serous ovarian cancer: association between quantitative CT features, residual tumour and disease progression within 12 months. Eur Radiol 28:4849–4859
Aerts HJ, Velazquez ER, Leijenaar RT et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Kumar V, Gu Y, Basu S et al (2012) Radiomics: the process and the challenges. Magn Reson Imaging 30:1234–1248
Nam KJ, Park H, Ko ES, Lim Y, Cho HH, Lee JE (2019) Radiomics signature on 3T dynamic contrast-enhanced magnetic resonance imaging for estrogen receptor-positive invasive breast cancers: preliminary results for correlation with Oncotype DX recurrence scores. Medicine (Baltimore) 98:e15871
Abbasian Ardakani A, Gharbali A, Saniei Y, Mosarrezaii A, Nazarbaghi S (2015) Application of texture analysis in diagnosis of multiple sclerosis by magnetic resonance imaging. Global J Health Sci 7:68–78
Lang N, Zhang Y, Zhang E et al (2019) Differentiation of spinal metastases originated from lung and other cancers using radiomics and deep learning based on DCE-MRI. Magn Reson Imaging. https://doi.org/10.1016/j.mri.2019.02.013
Qian Z, Li Y, Wang Y et al (2019) Differentiation of glioblastoma from solitary brain metastases using radiomic machine-learning classifiers. Cancer Lett 451:128–135
Fan M, Li H, Wang S, Zheng B, Zhang J, Li L (2017) Radiomic analysis reveals DCE-MRI features for prediction of molecular subtypes of breast cancer. PLoS One 12:e0171683
Calamante F (2013) Arterial input function in perfusion MRI: a comprehensive review. Prog Nucl Magn Reson Spectrosc 74:1–32
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Chikui T, Obara M, Simonetti AW et al (2012) The principal of dynamic contrast enhanced MRI, the method of pharmacokinetic analysis, and its application in the head and neck region. Int J Dent 2012:480659
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107
Park JE, Park SY, Kim HJ, Kim HS (2019) Reproducibility and generalizability in radiomics modeling: possible strategies in radiologic and statistical perspectives. Korean J Radiol 20:1124–1137
Kuhn M (2008) Building predictive models in R using the caret package. J Stat Softw 28:1–26
Christopher M (2006) Pattern recognition and machine learning, 1st edn. Springer, Bishop
Simundic AM (2009) Measures of diagnostic accuracy: basic definitions. EJIFCC 19:203–211
Bazot M, Nassar-Slaba J, Thomassin-Naggara I, Cortez A, Uzan S, Darai E (2006) MR imaging compared with intraoperative frozen-section examination for the diagnosis of adnexal tumors; correlation with final histology. Eur Radiol 16:2687–2699
Thomassin-Naggara I, Balvay D, Aubert E et al (2012) Quantitative dynamic contrast-enhanced MR imaging analysis of complex adnexal masses: a preliminary study. Eur Radiol 22:738–745
Zhang H, Mao Y, Chen X et al (2019) Magnetic resonance imaging radiomics in categorizing ovarian masses and predicting clinical outcome: a preliminary study. Eur Radiol 29:3358–3371
Kazerooni AF, Malek M, Haghighatkhah H et al (2017) Semiquantitative dynamic contrast-enhanced MRI for accurate classification of complex adnexal masses. J Magn Reson Imaging 45:418–427
Niu Q, Jiang X, Li Q et al (2018) Texture features and pharmacokinetic parameters in differentiating benign and malignant breast lesions by dynamic contrast enhanced magnetic resonance imaging. Oncol Lett 16:4607–4613
Monti S, Aiello M, Incoronato M et al (2018) DCE-MRI pharmacokinetic-based phenotyping of invasive ductal carcinoma: a radiomic study for prediction of histological outcomes. Contrast Media Mol Imaging 2018:5076269
Funding
Applied Basic Research Programs of Shanxi Province (201801D221116, 201701D121142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Jinliang Niu.
Conflict of interest
One of the authors of this manuscript (Jia-Liang Ren) is an employee of GE Healthcare. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Jia-Liang Ren) has significant statistical expertise.
Informed consent
Written informed consent was waived in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 354 kb)
Rights and permissions
About this article
Cite this article
Song, Xl., Ren, JL., Zhao, D. et al. Radiomics derived from dynamic contrast-enhanced MRI pharmacokinetic protocol features: the value of precision diagnosis ovarian neoplasms. Eur Radiol 31, 368–378 (2021). https://doi.org/10.1007/s00330-020-07112-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07112-0