Abstract
Objectives
Investigate the relationship between quantified terminal ileal (TI) motility and histopathological activity grading, Crohn Disease MRI Index (CDMI) and faecal calprotectin.
Methods
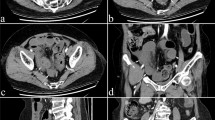
Retrospective review of children with Crohn disease or unclassified inflammatory bowel disease, who underwent MR enterography. Dynamic imaging for 25 patients (median age 12, range 5 to 16) was analysed with a validated motility algorithm. The TI motility score was derived. The primary reference standard was TI Endoscopic biopsy Assessment of Inflammatory Activity (eAIS) within 40 days of the MR enterography. Secondary reference standards: (1) the Crohn Disease MRI Index (CDMI) and (2) faecal calprotectin levels.
Results
MR enterography median motility score was 0.17 a.u. (IQR 0.12 to 0.25; range 0.05 to 0.55), and median CDMI was 3 (IQR 0 to 5.5). Forty-three percent of patients had active disease (eAIS > 0) with a median eAIS score of 0 (IQR 0 to 2; range 0 to 5). The correlation between eAIS and motility was r = − 0.58 (p = 0.004, N = 23). Between CDMI and motility, r = − 0.42 (p = 0.037, N = 25). Motility score was lower in active disease (median 0.12 vs 0.21, p = 0.020) while CDMI was higher (median 5 vs 1, p = 0.04). In a subset of 12 patients with faecal calprotectin within 3 months of MR enterography, correlation with motility was r = − 0.27 (p = 0.4).
Conclusions
Quantified terminal ileum motility decreases with increasing histopathological abnormality in children with Crohn disease, reproducing findings in adults. TI motility showed a negative correlation with an MRI activity score but not with faecal calprotectin levels.
Key Points
• It is feasible to perform MRI quantified bowel motility assessment in children using free-breathing techniques.
• Bowel motility in children with Crohn disease decreases as the extent of intestinal inflammation increases.
• Quantified intestinal motility may be a candidate biomarker for treatment efficacy in children with Crohn disease.






Similar content being viewed by others
Abbreviations
- a.u.:
-
Arbitrary units
- CDMI:
-
Crohn Disease MRI Index
- CRP:
-
C-reactive protein
- DRAM:
-
Dual Registration of Abdominal Motion
- eAIS:
-
Endoscopic Acute Inflammatory Score
- IBD:
-
Inflammatory bowel disease
- IQR:
-
Interquartile range
- MEGS:
-
Magnetic Resonance Enterography Global Score
- PACS:
-
Picture Archiving and Communication System
- SD:
-
Standard deviation
- SSFSE:
-
Steady-state fast spin echo
- TI:
-
Terminal ileum
- TNF:
-
Tumour necrosis factor
References
Menys A, Atkinson D, Odille F et al (2012) Quantified terminal ileal motility during MR enterography as a potential biomarker of Crohn’s disease activity: a preliminary study. Eur Radiol 22:2494–2501
Bickelhaupt S, Froehlich JM, Cattin R et al (2014) Software-assisted small bowel motility analysis using free-breathing MRI: feasibility study. J Magn Reson Imaging 39(1):17–23
Cullmann JL, Bickelhaupt S, Froehlich JM et al (2013) MR imaging in Crohn’s disease: correlation of MR motility measurement with histopathology in the terminal ileum. Neurogastroenterol Motil 25:749–e577
Menys A, Puylaert C, Tutein Nolthenius CE et al (2018) Quantified terminal ileal motility during MR enterography as a biomarker of Crohn disease activity: prospective multi-institution study. Radiology 289:428–435
Plumb AA, Menys A, Russo E et al (2015) Magnetic resonance imaging-quantified small bowel motility is a sensitive marker of response to medical therapy in Crohn’s disease. Aliment Pharmacol Ther 42:343–355
Puylaert CAJ, Nolthenius CJT, Tielbeek JAW et al (2019) Comparison of MRI activity scoring systems and features for the terminal ileum in patients with Crohn disease. AJR Am J Roentgenol 212(2):W25–W31
Vernier-Massouille G, Balde M, Salleron J et al (2008) Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology 135:1106–1113
Van Limbergen J, Russell RK, Drummond HE et al (2008) Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 135:1114–1122
Dubron C, Avni F, Boutry N, Turck D, Duhamel A, Amzallag-Bellenger E (2016) Prospective evaluation of free-breathing diffusion-weighted imaging for the detection of inflammatory bowel disease with MR enterography in childhood population. Br J Radiol 89:20150840
Menys A, Hamy V, Makanyanga J et al (2014) Dual registration of abdominal motion for motility assessment in free-breathing data sets acquired using dynamic MRI. Phys Med Biol 59:4603–4619
Barber JL, Zambrano-Perez A, Olsen ØE et al (2018) Detecting inflammation in inflammatory bowel disease — how does ultrasound compare to magnetic resonance enterography using standardised scoring systems? Pediatr Radiol 48(6):843–851
Odille F, Menys A, Ahmed A et al (2012) Quantitative assessment of small bowel motility by nonrigid registration of dynamic MR images. Magn Reson Med 68:783–793
Stange EF, Travis SPL, Vermeire S et al (2006) European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut 55(Suppl 1):i1–i15
Steward MJ, Punwani S, Proctor I et al (2012) Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol 81(9):2080–2088
Berni Canani R, Terrin G, Rapacciuolo L et al (2008) Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis 40(7):547–553
Robin X, Turck N, Hainard A et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. https://doi.org/10.1186/1471-2105-12-77
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Sun X, Xu W (2014) Fast implementation of DeLong’s algorithm for comparing the areas under correlated receiver operating characteristic curves. IEEE Signal Process Lett 21(11):1389–1393
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979(86):420–428
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 327(8476):307–310
Datta D, Love J (2017) Blandr: a Bland-Altman method comparison package for R. Zenodo. https://doi.org/10.5281/zenodo.824514
Zou KH, Hall WJ, Shapiro DE (1997) Smooth non-parametric receiver operating characteristic (ROC) curves for continuous diagnostic tests. Stat Med 16:2143–2156
Bithell J, Carpenter J (2000) Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 19:1141–1164
Menys A, Taylor SA, Emmanuel A et al (2013) Global small bowel motility: assessment with dynamic MR imaging. Radiology 269:443–450
Menys A, Makanyanga J, Plumb A et al (2016) Aberrant motility in unaffected small bowel is linked to inflammatory burden and patient symptoms in Crohn’s disease. Inflamm Bowel Dis 22(2):424–432
Gollifer RM, Menys A, Makanyanga J et al (2018) Relationship between MRI quantified small bowel motility and abdominal symptoms in Crohn’s disease patients-a validation study. Br J Radiol 91:20170914
Makanyanga JC, Pendsé D, Dikaios N et al (2014) Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol Eur Radiol 24:277–287
Bickelhaupt S, Pazahr S, Chuck N et al (2013) Crohn’s disease: small bowel motility impairment correlates with inflammatory-related markers C-reactive protein and calprotectin. Neurogastroenterol Motil 25(6):467–e363
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Acknowledgements
Motilent provided the GIQuant software free of charge for purposes of this study. The Centre for Medical Imaging, University College London, provided statistical support via HF and AM. This research project was supported by researchers (SAT) at the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Funding
The authors state that this work has not received any direct funding. SAT receives funding from the National Institute for Health Research University College London Hospitals Biomedical Research Centre. HF is funded by the Biotechnology and Biological Sciences Research Council (BBSRC) London Interdisciplinary Doctoral (LIDo) Consortium [BB/M009513/1] and receives funding from Motilent as part of an industrial placement.
Author information
Authors and Affiliations
Contributions
LC: collected and analysed clinical and histopathology data, and reviewed and edited the final manuscript
HF: provided statistical analysis and created all graphs and statistical diagrams, and reviewed and edited the final manuscript
AM: image analysis with TW and statistical analysis; reviewed and edited the early and final versions of the manuscript
TG: image analysis and reviewed and edited the final copy of the manuscript
SK: radiological data collection and analysis; reviewed and edited the final version of the manuscript
FK: clinical data collection; reviewed and edited the final version of the manuscript
DR and LP: histopathology data analysis; reviewed and edited the final version of the manuscript
SAT: supervised data analysis; reviewed and edited all versions of the manuscript including the final version
TW: corresponding author. TW supervised data collection, analysed the motility data, performed the MRI activity scoring, and prepared the final manuscript for publication
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is:
Professor Stuart Taylor, NIHR Senior Investigator and Consultant Radiologist, University College London, Centre for Medical Imaging.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
AM—CEO, Motilent
SAT—Motilent shareholder and paid consultant for Robarts
HF—funded for her PhD and for her industrial placement at Motilent by the Biotechnology and Biological Sciences Research Council (BBSRC) London Interdisciplinary Doctoral (LIDo) Consortium [BB/M009513/1]. She has received funding from Motilent to cover travel expenses to conferences as part of her industrial placement. She has had free use of Motilent GIQuant during her PhD.
The remaining authors declare no relevant conflicts of interest.
Statistics and biometry
Two of the authors have significant statistical expertise (HF and AM).
Informed consent
Written informed consent was waived by the Institutional Review Board. R&D Reference number 18BB10.
Ethical approval
Institutional Review Board approval was obtained. REC number 10/H0720/91GOSH.
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cococcioni, L., Fitzke, H., Menys, A. et al. Quantitative assessment of terminal ileum motility on MR enterography in Crohn disease: a feasibility study in children. Eur Radiol 31, 775–784 (2021). https://doi.org/10.1007/s00330-020-07084-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07084-1