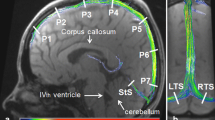
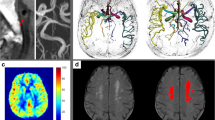
Abstract
Objectives
To use multi-parametric magnetic resonance imaging (MRI) to test the hypothesis that hypertensives would have higher retrograde venous blood flow (RVBF) in the internal jugular veins (IJV) vs. normotensives, and that this would inversely correlate with arterial inflow and gray matter, white matter, and cerebrospinal fluid volumes.
Methods
Following local institutional review board approval and written consent, a prospective observational 3-T MRI study of 42 hypertensive patients (53 ± 2 years, BMI 28.2 ± 0.6 kg/m2, ambulatory daytime systolic BP 148 ± 2 mmHg, ambulatory daytime diastolic BP 101 ± 2 mmHg) and 35 normotensive patients (48 ± 2 years, BMI 25.2 ± 0.8 kg/m2, ambulatory daytime systolic BP 119 ± 3 mmHg, ambulatory daytime diastolic BP 90 ± 2 mmHg) was performed. Phase contrast imaging calculated percentage retrograde venous blood flow (%RVBF), brain segmentation estimated regional brain volumes from 3D T1-weighted images, and pseudo-continuous arterial spin labeling measured regional cerebral blood perfusion. Statistical analysis included two-sample equal variance Student’s T tests, two-way analysis of variance with Tukey’s post hoc correction, and permutation-based two-group general linear modeling (p < 0.05).
Results
In the left IJV, %RVBF was higher in hypertensives (6.1 ± 1.5%) vs. normotensives (1.1 ± 0.3%, p = 0.003). In hypertensives, there was an inverse relationship of %RVBF (permutation-based general linear modeling) to cerebral blood flow in several brain regions, including the left occipital pole and the cerebellar vermis (p < 0.01). Percentage retrograde flow in the left IJV correlated inversely with the total matter volume (gray plus white matter volume) in hypertensives (r = − 0.49, p = 0.004).
Conclusion
RVBF in the left IJV is greater in hypertensives vs. normotensives and is linked to regional hypoperfusion and brain total matter volume.
Key Points
• Hypertensive humans have higher retrograde cerebral venous blood flow, associated with regional brain hypoperfusion and lower tissue volume, compared with controls.
• Cerebral retrograde venous blood flow may add further stress to already hypoperfused tissue in hypertensive patients.
• The amount of retrograde venous blood flow in hypertensive patients may predict which patients might be at higher risk of developing cerebral pathologies.



Similar content being viewed by others
Abbreviations
- BP:
-
Blood pressure
- CSA:
-
Cross-sectional area
- CSF:
-
Cerebrospinal fluid
- IJV:
-
Internal jugular vein
- MRI:
-
Magnetic resonance imaging
- PCASL:
-
Pseudo-continuous arterial spin labeling
- ROI:
-
Region of interest
- RVBF:
-
Retrograde venous blood flow
References
de Leeuw F-E, de Groot JC, Oudkerk M et al (2002) Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 125:765–772
Raz N, Lindenberger U, Rodrigue KM et al (2005) Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. https://doi.org/10.1093/cercor/bhi044
Muller M, van der Graaf Y, Visseren FL Mali WP, Geerlings MI(2012) Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol 71:825–833. https://doi.org/10.1002/ana.23554
Qiu C, Winblad B, Fratiglioni L (2005) The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 4:487–499. https://doi.org/10.1016/S1474-4422(05)70141-1
Warnert EAH, Rodrigues JCL, Burchell AE et al (2016) Is high blood pressure self-protection for the brain? Circ Res 119:e140–e151. https://doi.org/10.1161/CIRCRESAHA.116.309493
Farkas E, De Jong GI, Apró E Keuker JI, Luiten PG (2001) Calcium antagonists decrease capillary wall damage in aging hypertensive rat brain. Neurobiol Aging 22:299–309
Schaller B (2004) Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans. Brain Res Rev 46:243–260. https://doi.org/10.1016/j.brainresrev.2004.04.005
Marcotti S, Marchetti L, Cecconi P et al (2015) An anatomy-based lumped parameter model of cerebrospinal venous circulation: can an extracranial anatomical change impact intracranial hemodynamics? BMC Neurol 15:95. https://doi.org/10.1186/s12883-015-0352-y
Paksoy Y, Genç BO, Genç E (2003) Retrograde flow in the left inferior petrosal sinus and blood steal of the cavernous sinus associated with central vein stenosis: MR angiographic findings. AJNR Am J Neuroradiol 24:1364–1368
Uchino A, Nomiyama K, Takase Y et al (2007) Retrograde flow in the dural sinuses detected by three-dimensional time-of-flight MR angiography. Neuroradiology 49:211–215. https://doi.org/10.1007/s00234-006-0186-9
Zamboni P, Menegatti E, Bartolomei I et al (2007) Intracranial venous haemodynamics in multiple sclerosis. Curr Neurovasc Res 4:252–258
Cheng C-Y, Chang F-C, Chao A-C Chung CP, Hu HH (2013) Internal jugular venous abnormalities in transient monocular blindness. BMC Neurol 13:94. https://doi.org/10.1186/1471-2377-13-94
Mawuenyega KG, Sigurdson W, Ovod V et al (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330:1774. https://doi.org/10.1126/science.1197623
Chung C-P, Beggs C, Wang P-N et al (2014) Jugular venous reflux and white matter abnormalities in Alzheimer’s disease: a pilot study. J Alzheimers Dis 39:601–609. https://doi.org/10.3233/JAD-131112
Kuriyama N, Tokuda T, Miyamoto J et al (2008) Retrograde jugular flow associated with idiopathic normal pressure hydrocephalus. Ann Neurol 64:217–221. https://doi.org/10.1002/ana.21410
Mancia G, Fagard R, Narkiewicz K et al (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34:2159–2219. https://doi.org/10.1093/eurheartj/eht151
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20:45–57. https://doi.org/10.1109/42.906424
Heiberg E, Sjögren J, Ugander M et al (2010) Design and validation of segment--freely available software for cardiovascular image analysis. BMC Med Imaging 10:1. https://doi.org/10.1186/1471-2342-10-1
Schrauben EM, Johnson KM, Huston J et al (2014) Reproducibility of cerebrospinal venous blood flow and vessel anatomy with the use of phase contrast-vastly undersampled isotropic projection reconstruction and contrast-enhanced MRA. AJNR Am J Neuroradiol 35:999–1006. https://doi.org/10.3174/ajnr.A3779
Buxton RB, Frank LR, Wong EC et al (1998) A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 40:383–396
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25
Winkler AM, Ridgway GR, Webster MA et al (2014) Permutation inference for the general linear model. Neuroimage 92:381–397. https://doi.org/10.1016/j.neuroimage.2014.01.060
Canhão P, Ferro JM, Stam J (2009) Cerebral venous thrombosis. Handb Clin Neurol 93:809–822. https://doi.org/10.1016/S0072-9752(08)93040-2
Sander D, Winbeck K, Etgen T et al (2000) Disturbance of venous flow patterns in patients with transient global amnesia. Lancet 356:1982–1984. https://doi.org/10.1016/S0140-6736(00)03313-4
Chung C-P, Wang P-N, Wu Y-H et al (2011) More severe white matter changes in the elderly with jugular venous reflux. Ann Neurol 69:553–559. https://doi.org/10.1002/ana.22276
Kiliç T, Akakin A (2008) Anatomy of cerebral veins and sinuses. Front Neurol Neurosci 23:4–15. https://doi.org/10.1159/000111256
Ono T, Okita Y, Ando M, Kitamura S (2000) Retrograde cerebral perfusion in human brains. Lancet 356:1323. https://doi.org/10.1016/S0140-6736(00)02818-X
Kawasaki R, Cheung N, Wang JJ et al (2009) Retinal vessel diameters and risk of hypertension: the Multiethnic Study of Atherosclerosis. J Hypertens 27:2386–2393. https://doi.org/10.1097/HJH.0b013e3283310f7e
Frost S, Kanagasingam Y, Sohrabi H et al (2013) Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry 3:e233. https://doi.org/10.1038/tp.2012.150
Gotoh M, Ohmoto T, Kuyama H (1993) Experimental study of venous circulatory disturbance by dural sinus occlusion. Acta Neurochir 124:120–126
Bedfor THB (1935) The effect of increased intracranial venous pressure on the pressure of the cerebrospinal fluid. Brain 58:427–447. https://doi.org/10.1093/brain/58.4.427
Welch K, Friedman V (1960) The cerebrospinal fluid valves. Brain 83:454–469
van der Veen PH, Muller M, Vincken KL et al (2014) Brain volumes and risk of cardiovascular events and mortality. The SMART-MR study. Neurobiol Aging 35:1624–1631. https://doi.org/10.1016/j.neurobiolaging.2014.02.003
Waki H, Bhuiyan MER, Gouraud SS et al (2011) Acute reductions in blood flow restricted to the dorsomedial medulla induce a pressor response in rats. J Hypertens 29:1536–1545. https://doi.org/10.1097/HJH.0b013e3283484106
San Millán Ruíz D, Gailloud P, Rüfenacht DA et al (2002) The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol 23:1500–1508
Kudo K, Terae S, Ishii A et al (2004) Physiologic change in flow velocity and direction of dural venous sinuses with respiration: MR venography and flow analysis. AJNR Am J Neuroradiol 25:551–557
Acknowledgments
The authors would like to thank Peter Hobden, Martin Stuart, and John Evans for their help in designing MR paradigms and acquiring the MR images. We would also like to thank the research nurses Rissa Calsena and Jenny Wilcox for their help in recruiting and screening the participants. Finally, we would like to thank the volunteers for participating in this study.
Funding
The study was funded by the British Heart Foundation (IBSRF FS/11/1/28400; ECH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Emma Hart.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2474 kb)
Rights and permissions
About this article
Cite this article
Rodrigues, J.C.L., Strelko, G., Warnert, E.A.H. et al. Retrograde blood flow in the internal jugular veins of humans with hypertension may have implications for cerebral arterial blood flow. Eur Radiol 30, 3890–3899 (2020). https://doi.org/10.1007/s00330-020-06752-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06752-6