Abstract
Objectives
To clarify the prevalence and risk factors of ascending aortic (AA) dilatation according to ESC 2014 guidelines.
Methods
This study included 1000 consecutive patients scheduled for diagnostic coronary artery computed tomographic angiography. AA diameter was retrospectively measured in 3 planes: sinus valsalva, sinotubular junction, and tubular part. The threshold for AA dilatation was set to > 40 mm which has been suggested as an upper normal limit for AA diameter in ESC 2014 guidelines on aortic diseases. Aortic size index (ASI) using the ratio between aortic diameter and body surface area (BSA) was applied as a comparative measurement. The threshold for AA dilatation was set to the upper limit of normal distribution exceeding two standard deviations (95%). Risk factors for AA dilatation were collected from medical records.
Results
The patients’ mean age was 52.9 ± 9.8 years (66.5% women). The prevalence of AA dilatation was 23.0% in the overall study population (52.5% males) and 15.1% in the subgroup of patients with no coronary artery disease or bicuspid (BAV)/mechanical aortic valve (n = 365). According to the normal-distributed ASI values, the threshold for sinus valsalva was defined as 23.2 mm/m2 and for tubular part 22.2 mm/m2 in the subgroup. Higher BSA was associated with larger AA dimensions (r = 0.407, p < 0.001). Male gender (p < 0.001), BAV (p < 0.001), hypertension (p = 0.009) in males, and smoking (p < 0.001) appeared as risk factors for AA dilatation.
Conclusions
The prevalence of AA dilatation is high with current ESC guidelines for normal AA dimension, especially in males. Body size is strongly associated with AA dimensions; it would be more reliable to use BSA-adjusted AA diameters for the definition of AA dilatation.
Key Points
• The prevalence of AA dilatation is high in patients who are candidates for coronary CT angiography.
• Body size is strongly associated with AA dimensions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European Society of Cardiology (ESC) guidelines suggest normal ascending aortic (AA) dimensions to be 40 mm or less in healthy adults. According to these criteria, patients with AA over 40 mm accompanied with risk factors should be monitored regularly either by computed tomography (CT) or magnetic resonance imaging (MRI) [1]. The recommended limit for surgical intervention in AA is 55 mm in cases with a normal aortic valve anatomy without inherited aortic disease [1, 2]. It has been demonstrated that the risk of aortic dissection or rupture increases considerably above this value [3].
The diameter of thoracic aorta and thoracic aortic dilatation (TAD) have been associated with increased age, male gender, and increased body surface area (BSA) [1]. Furthermore, it has been related to hypertension and smoking [4,5,6]. The hemodynamic conditions in the aorta play a significant role in determining TAD [7]. In particular, the bicuspid aortic valve (BAV) often causes valvular dysfunction (stenosis and regurgitation) and abnormal flow in the ascending aorta. BAV is associated strongly with TAD [8]. The prevalence of BAV in Western and Caucasian populations has varied from 0.5 to 10.9% depending on the study and study population [9,10,11]. Furthermore, the prevalence of TAD has been reported to range from 30% up to even 70% in patients with BAV [12,13,14].
The aim of this study was to determine the prevalence of AA dilatation according to ESC 2014 guidelines and to clarify AA dilatation risk factors in a consecutive single-center population scheduled for coronary CT angiography (CCTA). Our secondary aim was to compare ESC guidelines’ stratifications with a classification adjusted for the patient’s body size.
Materials and methods
Patient population
This retrospective study examined 1065 consecutive patients with low to moderate pretest probability for coronary artery disease (CAD) and without pre-existing aortic diseases who had been imaged with CCTA between January 2012 and March 2018. Sixty-four patients were excluded due to motion artifacts or inadequate visibility of AA in CCTA and one patient due to age under 16 years. Patients’ baseline characteristics are presented in Table 1.
From this total study population (n = 1000), we selected for further analyses a subgroup with (1) no CAD (over 50% stenosis or coronary calcification) in CCTA, (2) no history of hypertension, or (3) no BAV or mechanical aortic valve. We named the subgroup “subgroup of patients with no risk factors.”
CCTA imaging procedure
CCTA imaging was performed during mid-diastole according to routine clinical practice using four different CT scanners capable of ECG-gated fast coronary imaging (Somatom Definition AS 64; Somatom Definition AS+ 128; Definition Edge; Definition Flash, Siemens Medical Solutions). The slice thickness of 0.6 mm was used in all scanners. Collimation was 64 × 0.6 mm with the Somatom Definition AS 64, and 128 × 0.6 mm for the other scanners [15]. The patients were scanned in the supine position with their hands above their head to avoid artifacts. With 64- and 128-slice scanners, bolus tracking was used to optimize the timing of the coronary scanning. When using the dual-energy scanner (Definition Flash), a 10-ml contrast agent test bolus was injected prior to actual imaging to evaluate the optimal timing for coronary scanning. Depending on the scanner, the contrast agent volume varied between 60 and 80 ml (Omnipaque 350 mg/ml, GE Healthcare). The infusion rate was 5 ml/s followed by 30 ml of a saline chaser. The tube voltage was adjusted according to the patient’s size, varying between 80 and 120 kV. Tube current modulation was applied for every patient. The image area extended from the tracheal bifurcation to the inferior cardiac apex. The in-plane resolution was 512 × 512 pixels, with z-axis coverage including the area from the bifurcation to the diaphragm. Prospective ECG gating was applied during helical scanning. The heart rate was optimized to be below 65 beats/min by administering 5–20 mg metoprolol succinate intravenously (Seloken, AstraZeneca AB) [15].
Data assessment
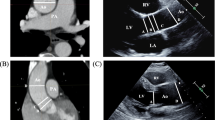
One observer retrospectively analyzed the CCTA images on an IDS7 diagnostic workstation (version17.3.6; Sectra Imtec). The slice thickness of the CCTA images was 0.6 mm. The ascending aorta was divided into 3 planes: sinus valsalva, sinotubular junction, and tubular part (Fig. 1). According to current recommendations, aortic diameters were measured from the outer-to-outer vascular wall perpendicular to the centerline of the vessel [1]. The largest of the three dome of the cusp to dome of the cusp diameters in sinus valsalva and the largest of the two diameters in sinotubular junction and tubular part were registered. Aortic valve anatomy (tricuspid, bicuspid, or mechanical aortic valve prosthesis), middle diastolic diameter of cardiac left ventricle (LV), area of left atrium (LA), thickness of left ventricular posterior wall, and interventricular septum were registered.
a Diameters of ascending aorta were measured in three planes: sinus valsalva (I), sinotubular junction (II), and tubular part (III). b The diameter of sinus valsalva was assessed as the largest of the three dome of the cusp to dome of the cusp diameters from the outer layer to the outer layer of the aortic wall (arrows). c, d The largest of the two diameters (1 and 2) of sinotubular junction and tubular part was measured as perpendicular to each other
AA dilatation classification methods
Several published classification methods were first applied to assess the frequencies of AA dilatation: the ESC 2014 recommendations (ESC Diameter) [1], Roman’s classification (aortic size index, ASI) [4], and aortic height index (AHI). The thresholds for aortic dilatation with each method were based on the values in the literature. After stratifying patients in the overall population into dilated or non-dilated groups, similar stratifications were made in the subgroup of patients with no risk factors.
In ESC Diameter, the AA was considered dilated if its greatest diameter exceeded 40 mm in any of the three measurement planes [1].
To estimate the ASI, we calculated the relationship between AA and BSA using Mosteller’s equation [16]; \( \frac{\mathrm{Aortic}\ \mathrm{diameter}\ \left(\mathrm{mm}\right)}{\mathrm{BSA}\ \left({\mathrm{m}}^2\right)} \) [17]. In our study, data on height was available for 775 patients, and on weight for 740 patients at the time of CCTA. Thus, BSA could be calculated in 740 patients. For both genders, the upper limit for ASI was 21 mm/m2 at the sinus valsalva plane according to Roman et al [4].
Furthermore, we calculated AHI to represent the relationship between aortic size and patient height: \( \frac{\mathrm{Aortic}\ \mathrm{diameter}\ \left(\mathrm{m}\mathrm{m}\right)}{\mathrm{patient}\ \mathrm{height}\ \left(\mathrm{m}\right)} \), which has been reported to evaluate satisfactorily the risk of complications in patients with ascending aortic aneurysms [18].
Finally, based on aortic diameters and the calculated ASI and AHI values, we arbitrarily defined an upper threshold of two standard deviations (2 SD, 95%) above the mean value as the upper limit of normal aortic diameter in the subgroup of patients with no risk factors. These thresholds were named as Aortic Diameter2SD, ASI2SD, and AHI2SD, and they were subsequently used to calculate the prevalence of increased aortic diameter in the overall study population.
Risk factors
Risk factors for cardiovascular diseases as well as other baseline characteristics were collected from medical records. The patient was defined as hypertensive if he/she was receiving medication for hypertension and diabetic if the patient had two separate fasting plasma glucose levels ≥ 7.0 mmol/l or ≥ 11 mmol/l in a glucose tolerance test or HbA1C ≥ 48 mmol/l. Diabetes was not subdivided into subtypes. Current smokers and those who had stopped continuous smoking less than 30 years ago were considered smokers. Based on the coronary artery findings in the CCTA, the patients were dichotomized as positive or negative in terms of CAD. CCTA reports were prepared by imaging cardiologists or cardiac radiologists with over 6 years of experience in cardiac imaging. Hypercholesterolemia was determined according to Finnish national recommendations as high LDL (> 3 mmol/l) and low HDL (males < 1 mmol/l, females < 1.2 mmol /l) concentrations.
Statistical analysis
The normality of the aortic dimension data was analyzed using the Kolmogorov–Smirnov test. Correlations between the diameters of AA and continuous scaled parameters were tested using Spearman correlation test. Continuous parameters were tested with the Mann–Whitney test with the results being presented as median values with variable range. Chi-square test was used for nominal parameters and results are presented as numbers and percentages. McNemar’s test was used to compare two dependent dichotomic variables. Statistical significance was set to p < 0.05 and high statistical significance to p < 0.001. All statistical analyses were performed by using SPSS Statistics 23 (IBM).
Ethical aspects
The study was approved by the local ethical committee. CCTA imaging had been performed on the basis of clinical indications; thus, the study caused no additional radiation dose to the patients. The patients’ clinical treatment was completely unaffected by the study.
Results
Baseline characteristics are shown in Table 1. The mean age of the overall study population was 52.9 ± 9.8 years and a majority of the patients were women (n = 665, 66.5%). The mean age of the subgroup of patients with no risk factors (n = 260 females, 71.2%) was 50.1 ± 10.8 years and their mean BSA value was 1.9 ± 0.2 m2.
According to the ESC guidelines, 230 patients were stratified as having a dilated AA when the measurement results from all three levels were combined. Thus, the overall prevalence of AA dilatation was 23.0% in the overall study population (8.1% in females and 52.5% in males) (Table 2). The prevalence of dilatation in the area of aortic root ranged from 5.1% in females up to 50.4% in males and in the tubular part from 4.7% in females up to 14.9% in males.
According to Roman’s classification, in the 740 patients for whom we had access to their height and weight data in medical records, 163 patients (23.0% of males and 21.5% of females) were stratified as having a dilated aortic root in the sinus valsalva plane. Thus, the prevalence of dilatation was 22.0% in both genders.
When the calculated prevalence of dilatation in the sinus valsalva plane was compared only in the male patients in our consecutive population, the prevalence was significantly higher when the ESC criteria were utilized in comparison to Roman’s method (p < 0.001). In contrast, in the female patients, the prevalence was significantly lower when the ESC criteria were compared with Roman’s method (p < 0.001).
In the subgroup of patients with no risk factors, the overall prevalence of AA dilatation was 15.1% (5.4% in females and 39.0% in males) according to the ESC recommendations. The prevalence of dilatation in aortic root ranged from 3.1% in females up to 37.1% in males and in the tubular part from 3.1% in females up to 7.6% in males.
Based on the values assessed in the subgroup of patients with no risk factors, thresholds for Aortic Diameter2SD, ASI2SD, and AHI2SD are presented in Table 3. With all measured planes combined, the frequencies of AA dilatation in the overall study population were 14.5% according to the Aortic Diameter2SD, 10.3% when based on ASI2SD, and 15.7% when determined with AHI2SD (Table 2).
Associations between the baseline characteristics and clinical risk factors with respect to the prevalence of aortic dilatation are shown in Table 4. According to the ESC criteria, BSA was associated with larger AA diameters in the overall population (r = 0.407, p < 0.001) and in the subgroup of patients with no risk factors (r = 0.405, p < 0.001). Furthermore, according to the ESC criteria, male gender was associated with AA dilatation (p < 0.001), i.e., 176 males (76.5%) but only 54 females (23.5%) had dilated AA. Other factors associated with dilated AA were hypertension in females (p = 0.009), BAV (p < 0.001), increased LV wall thickness (p < 0.001), increased middle diastolic LV diameter (p < 0.001), and increased LA area (p < 0.001), Table 4. The association between the diameter of sinus valsalva and the patient’s height is illustrated in Fig. 2. According to the ESC criteria, if the patient was taller than 180 cm, this increased significantly the prevalence of dilatation in his/her sinus valsalva plane (61.8%) compared with patients whose height was less than 180 cm (16.8%, p < 0.001).
Association between the diameter of the sinus valsalva and the patient’s height. The ESC recommendation for the upper limit of a normal aorta (40 mm) is shown by the red reference line. The linear correlation (r = 0.535, p < 0.001) between the diameter of the sinus valsalva and the patient’s height is shown by the black line
When using the ASI2SD classification, gender was not associated with the prevalence of AA, but using AHI2SD, gender was associated with AA dilatation (p < 0.001) (Table 4). Also, increased LV wall thicknesses (p = 0.001), LV middle diastolic diameter (p = 0.001), and increased LA area (p < 0.001) were associated with AA dilatation using AHI2SD classification (Table 4).
Discussion
When applying the current ESC guidelines for aortic dilatation, the prevalence of AA dilatation was as high as 23% in our consecutive CCTA population. In males, this prevalence exceeded 50% and in male patients taller than 180 cm, the prevalence exceeded 60%. Even in those patients without hypertension, coronary artery disease, or bicuspid or mechanical aortic valve, the prevalence still remained as high as 15%. Our results indicate that the ESC guidelines may overestimate the prevalence of aortic dilatation and might lead to unwarranted distress in patients who may be subjected to unnecessary follow-up examinations by either CT or MRI. Furthermore, a greater BSA is moderately associated with larger AA diameters. BSA-adjusted classification is a level of evidence B in ESC guidelines. Thus, when diagnosing aortic dilatation, it might be better to use BSA-adjusted classifications.
Aortic dilatation is one of the main causes for repeated imaging and clinical follow-up. The additional follow-up imaging caused by unreliable classification levels burdens the healthcare system and increases costs. The mean age of our patient cohort was 53 years indicating that every second male patient would require follow-up imaging for decades should the ESC recommendations be followed. In general, the limits for AA dilatation should be based on the risk for aortic complications: rupture, dissection, or death. The incidence of rupture of thoracic aortic aneurysm is 5/100,000 (0.005%) [19]. Davies et al demonstrated that thoracic aortic diameters in the range 35–39 mm are not associated with aortic ruptures or dissections [20]. However, in that same study, a thoracic aortic diameter of 60 mm or over was related to a dramatic risk of either rupture or dissection, i.e., the odds ratio for rupture increased by 27-fold.
The incidence of AA dilatation in the North American population has been shown to be approximately 10/100,000 inhabitants (0.01%) and it was similar in both genders [21]. Compared with these previous data, the prevalence values estimated in our study population seem extremely high.
ESC guidelines do not present rigorous thresholds to the upper limit of AA diameter, but it states that aortic diameters do not normally exceed 40 mm in healthy adults, which is based on published data [1].
Previously, Roman et al have suggested that the upper limit for normal sinus valsalva diameter would be 21 mm/m2 using ASI [4]. They calculated this limit on the basis of a study population consisting of 135 adults. The corresponding upper limit in our study was slightly higher (23 mm/m2) when using the ASI2SD threshold determined from our subgroup of patients with no risk factors. For comparison, in their MRI study, Mensel et al postulated that the normal upper limit for the tubular part would be 42 mm for males and 39 mm for females [22]. Our results parallel the thresholds published by Mensel et al as the normal upper limit for tubular part in our study was 43 mm for males and 39 mm for females when using the AA diameter2SD assessment. Mensel et al did not examine the aortic root diameters [22].
Similar to previous reports, the associated risk factors for AA dilatation in the present study were male gender, BAV, hypertension, and smoking [4,5,6]. Hypertension is only associated with AA dilatation in males. The diameters of sinus valsalva and tubular part also correlated with the patient’s height, weight, and therefore also with BSA. BSA has also been observed to correlate positively with AA dilatation [4, 6, 23]. In the present study, increased LV wall thickness was also associated with aortic dilatation when applying the ESC recommendations. Aortic root dilatation may lead to aortic regurgitation and it has also been reported to be associated with LV hypertrophy, LV dilatation, and LV dysfunction [24]. However, when using ASI2SD thresholds, LV thickness is not associated with AA dilatation.
Male gender is associated with high statistical significance to AA dilatation when using 40 mm as the upper limit of a normal AA in agreement with previous studies [6]. However, gender is not associated with the prevalence of AA dilatation when using the ASI2SD method due to the fact that BSA is strongly linked with gender (i.e., men tend to be taller and heavier than women).
The main limitations of this retrospective study were the higher number of females and that only a limited part of the AA was available for analysis due to imaging stack disposition. In addition, a relatively high number of patients had risk factors for cardiovascular diseases. However, all patients had only a low to moderate pretest probability for CAD and the patients’ mean age was 52.9 years with a relatively low standard deviation (9.8 years), which means that this population was representative of clinical CCTA populations in many hospitals. Intra- and interobserver reproducibility analyses for measurements of AA diameter were not performed in the present study. However, in our prior study with 1.5-T aortic magnetic resonance angiography, interobserver reproducibility was shown to be excellent (ICC = 0.917) with 20 dilated AA and 20 non-dilated AA patients [7]. Lu et al showed in a small 30-patient CT population that the maximum variability was 1.2 mm in the measured AA diameter and low variability indicated that AA diameter is a reliable measurement [25]. ECG-gated CT is an important tool for precise measurements of AA diameter.
In conclusion, the prevalence of thoracic aortic dilatation proved to be relatively high in this consecutive CCTA population if they were assessed with the ESC 2014 guidelines. This has significant clinical consequences, since patients whose values lie outside the normal limits are usually scheduled for repeated follow-up for the rest of their lives. Based on this study and ESC guidelines, we propose that a more reliable way to evaluate thoracic aortic diameter would be indexing the AA diameter to body size by calculating it in terms of BSA. By using indexed upper limits for AA, the specificity of the stratifications would increase, and repeated follow-up might be targeted to those patients who will truly benefit. However, more clinical studies will be needed to determine the optimal normal limits for AA dilatation in different patient populations.
Abbreviations
- 2 SD:
-
Two standard deviations
- AA:
-
Ascending aorta
- AHI:
-
Aortic height index
- ASI:
-
Aortic size index
- BAV:
-
Bicuspid aortic valve
- BSA:
-
Body surface area
- CAD:
-
Coronary artery disease
- CCTA:
-
Coronary computed tomography angiography
- CT:
-
Computed tomography
- ESC:
-
European Society of Cardiology
- LA:
-
Left atrium
- LV:
-
Left ventricle
- MRI:
-
Magnetic resonance imaging
- TAD:
-
Thoracic aortic dilatation
References
Erbel R, Aboyans V, Boileau C et al (2014) 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 35(41):2873–2926
Hiratzka LF, Bakris GL, Beckman JA et al (2010) 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Anesth Analg 111(2):279–315
Chau KH, Elefteriades JA (2013) Natural history of thoracic aortic aneurysms: size matters, plus moving beyond size. Prog Cardiovasc Dis 56(1):74–80
Roman MJ, Devereux RB, Kramer-Fox R, O’Loughlin J (1989) Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 64(8):507–512
Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC (1991) Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 13(3):452–458
Rogers IS, Massaro JM, Truong QA et al (2013) Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study). Am J Cardiol 111(10):1510–1516
Kauhanen SP, Hedman M, Kariniemi E et al (2019) Aortic dilatation associates with flow displacement and increased circumferential wall shear stress in patients without aortic stenosis: a prospective clinical study. J Magn Reson Imaging 50(1):136–145
Losenno KL, Goodman RL, Chu MWA (2012) Bicuspid aortic valve disease and ascending aortic aneurysms: gaps in knowledge. Cardiol Res Pract 2012
Basso C, Boschello M, Perrone C et al (2004) An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol 93(5):661–663
Pauperio HM, Azevedo AC, Ferreira CS (1999) The aortic valve with two leaflets--a study in 2,000 autopsies. Cardiol Young 9(5):488–498
Wang X, Wang W (2017) Prevalence of bicuspid aortic valve in Chinese patients with aortic valve disease: a systematic review. J Heart Valve Dis 26(3):274–280
Ferencik M, Pape LA (2003) Changes in size of ascending aorta and aortic valve function with time in patients with congenitally bicuspid aortic valves. Am J Cardiol 92(1):43–46
Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G (1999) Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart 82(1):19–22
Novaro GM, Tiong IY, Pearce GL, Grimm RA, Smedira N, Griffin BP (2003) Features and predictors of ascending aortic dilatation in association with a congenital bicuspid aortic valve. Am J Cardiol 92(1):99–101
Korhonen M, Mustonen P, Hedman M et al (2018) Left atrial appendage morphology and relative contrast agent concentration in patients undergoing coronary artery CTA. Clin Radiol 73(11):982.e17-982
Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317(17):1098
Davies RR, Gallo A, Coady MA et al (2006) Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 81(1):169–177
Zafar MA, Li Y, Rizzo JA et al (2018) Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg 155(5):1938–1950
Johansson G, Markström U, Swedenborg J (1995) Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. J Vasc Surg 21(6):985–988
Davies RR, Goldstein LJ, Coady MA et al (2002) Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 73(1):28
Elefteriades JA, Sang A, Kuzmik G, Hornick M (2015) Guilt by association: paradigm for detecting a silent killer (thoracic aortic aneurysm). Open Heart 2(1):000169 eCollection 2015
Mensel B, Heßelbarth L, Wenzel M et al (2016) Thoracic and abdominal aortic diameters in a general population: MRI-based reference values and association with age and cardiovascular risk factors. Eur Radiol 26(4):969–978
Campens L, Demulier L, De Groote K et al (2014) Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am J Cardiol 114(6):914–920
Roman MJ, Devereux RB, Niles NW et al (1987) Aortic root dilatation as a cause of isolated, severe aortic regurgitation. Prevalence, clinical and echocardiographic patterns, and relation to left ventricular hypertrophy and function. Ann Intern Med 106(6):800–807
Lu TC, Rizzo E, Marques-Vidal PM, Segesser LKV, Dehmeshki J, Qanadli SD (2010) Variability of ascending aorta diameter measurements as assessed with electrocardiography-gated multidetector computerized tomography and computer assisted diagnosis software. Interact Cardiovasc Thorac Surg 10(2):217–221
Acknowledgements
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital.
Funding
This study has received funding from Oiva Vaittinen Will Grant, Aarne Koskelo Foundation, Finnish Society of Angiology, Radiological Society of Finland, Northern Savonia Foundation of the Finnish Cultural Foundation, Finnish Foundation of Cardiovascular Research, and Ida Montin Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Marja Hedman.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Statistician Tuomas Selander kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was not required for this study because this was a retrospective study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Korhonen M, Mustonen P, Hedman M, Vienonen J, Onatsu J, Vanninen R, et al Left atrial appendage morphology and relative contrast agent concentration in patients undergoing coronary artery CTA. Clin Radiol 2018 Jul 18.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kauhanen, S.P., Saari, P., Jaakkola, P. et al. High prevalence of ascending aortic dilatation in a consecutive coronary CT angiography patient population. Eur Radiol 30, 1079–1087 (2020). https://doi.org/10.1007/s00330-019-06433-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06433-z