Abstract
There is yet no consensus on the application of functional imaging and qualitative image interpretation in the management of gastric cancer. In this second part, we will discuss the role of image-derived quantitative parameters from dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in gastric cancer, as both techniques have been shown to be promising and useful tools in the clinical decision making of this disease. We will focus on different aspects including aggressiveness assessment, staging and Lauren type discrimination, prognosis prediction and response evaluation. Although both the number of articles and the patients enrolled in the studies were rather small, there is evidence that quantitative parameters from DCE-MRI such as Ktrans, Ve, Kep and AUC could be promising image-derived surrogate parameters for the management of gastric cancer. Data from 18F-FDG PET/CT studies showed that standardised uptake value (SUV) is significantly associated with the aggressiveness, treatment response and prognosis of this disease. Along with the results from diffusion-weighted MRI and contrast-enhanced multidetector computed tomography presented in Part 1 of this critical review, there are additional image-derived quantitative parameters from DCE-MRI and 18F-FDG PET/CT that hold promise as effective tools in the diagnostic pathway of gastric cancer.
Key Points
• Quantitative analysis from DCE-MRI and18F-FDG PET/CT allows the extrapolation of multiple image-derived parameters.
• Data from DCE-MRI (Ktrans, Ve, Kep and AUC) and 18F-FDG PET/CT (SUV) are non-invasive, quantitative image-derived parameters that hold promise in the evaluation of the aggressiveness, treatment response and prognosis of gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most common malignancies worldwide [1]. As already discussed in the first part (Part 1) of this critical review [2], this disease is managed through a standardised multidisciplinary approach where radiology plays a crucial role in the detection, staging, treatment planning and follow-up [3, 4].
The most useful techniques are endoscopic ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET)/CT. At this regard, the PLASTIC trial [5] is an ongoing study that will evaluate the impact and cost-effectiveness of PET and staging laparoscopy in addition to initial staging in patients with locally advanced GC.
Different image-derived quantitative parameters from these techniques could be considered promising tools in the management of GC [6, 7], as they reflect a variety of biological processes (normal or pathological) both at baseline and after therapeutic interventions.
Quantitative imaging has the potential to improve the value of diagnostic testing and enhance clinical productivity and is increasingly important in preclinical studies, clinical research, and clinical practice [7]. Oncological imaging represents an ideal setting for the collection of new image-derived quantitative parameters from different techniques that can be potentially included in the clinical scenario [6]. The Radiological Society of North America underlined their importance as non-invasive tools with different applications in oncology and has promoted their use in clinical trials [7].
In the second part, we will provide a critical review on the state of the art of dynamic contrast-enhanced (DCE) MRI and 18F-FDG PET/CT findings.
Evidence acquisition
We searched MEDLINE/PubMed for manuscripts published from inception to 17 August 2018 (Fig. 1).
DCE-MRI and image-derived quantitative parameters
DCE-MRI is a functional imaging technique in which multiphase images are acquired over a few minutes at baseline, during and after rapid intravenous injection of a contrast agent and a saline flush. Changes in signal intensity (reflecting tissue vascularity) can be observed and parametric maps of specific microvascular image-derived quantitative parameters can be derived [8, 9]. Basic recommendations include an adequate spatial/temporal resolution and knowledge of the inherent characteristics of the contrast agent. Semi-quantitative and quantitative analysis can be performed on specific regions of interest (ROIs) or on a pixel-by-pixel basis.
DCE-MRI requires high temporal resolution (usually 4–6 s/phase) and can be degraded by motion artefacts (e.g. respiratory or bowel peristalsis) [10]. Therefore, an injection of intravenous/intramuscular anti-peristaltic agent is advised to reduce the mobility of the gastric walls.
DCE-MRI reflects tumour angiogenesis (i.e. the creation of new blood vessels) and is directly associated with tumour growth and inversely correlated with prognosis [11,12,13].
Different quantitative parameters can be extrapolated from DCE-MRI maps (Tofts model) [14] such as:
Ktrans (min−1): volume transfer constant of gadolinium from blood plasma to the extravascular extracellular space (EES)
Ve (0 to 100%): volume of the EES per unit volume of tissue (i.e. the amount of “space” available within the interstitium for accumulating gadolinium)
Kep (min−1): rate constant gadolinium reflux from the EES back into the vascular system (i.e. it is the ratio: Ktrans/Ve)
AUC (mmol/s): area under the gadolinium concentration curve during a certain period of time.
The application of DCE-MRI in GC has been increasingly growing over the last few years thanks to the technical developments (e.g. the shortening of temporal resolution) and the advantage of free-from-radiation damage compared with CT.
Although certainly interesting in a research context, this technique has been mainly applied for neuro-oncological imaging so far. However, DCE-MRI in organ systems outside the central nervous system for oncological applications remains an active area of research, especially for breast, liver and prostate cancer. Other applications of DCE-MRI have been investigated, but as yet are not routinely used in clinical practice for GC. A possible explanation is that tumours are biologically complex structures and, differently from other organs such as the brain, the DCE-MRI protocols for GC are flawed by the presence of several artefacts (especially due to peristalsis) that can easily undermine the quality of the scan and the interpretation of quantitative data from the regions of interest analysed.
DCE-MRI in the detection and diagnosis of gastric cancer
Table 1 summarises the main studies analysing the role of DCE-MRI in GC.
The first study by Kang and colleagues dates back to 2000 [15] and reports the usefulness of dynamic and delayed MRI for T staging. The thickness and enhancement pattern of normal and pathological gastric walls were compared in 46 patients through a dynamic protocol including precontrast images and additional acquisitions of 30, 60, 90 and 240–300 s after injection of gadolinium. The pathological outer layers (mucosa and submucosa) showed earlier enhancement (i.e. between 30 and 90 s) than the normal gastric wall in 43/46 patients (93%) and the peak enhancement of the normal gastric wall was > 90 s in 17/46 patients (37%). A reasonable high consistency between MR staging and pathological staging for all T stages was reported (accuracy for T stage, 83%). Such results, although not related to any specific quantitative parameter, show that dynamic MR imaging was already a promising technique for predicting T staging in GC at that time.
Joo and colleagues [16] correlated DCE-MRI parameters with prognostic factors such as pathological T staging and epidermal growth factor receptor (EGFR) expression. Ve and iAUC were significantly higher for GC (0.133 and 5.533 mmol/s, respectively) when compared with normal gastric wall (0.063 and 3.894, respectively) (all p < 0.05). Additionally, Ve was positively correlated with T staging (ρ = 0.483, p = 0.023) and Ktrans was significantly correlated with EGFR expression (ρ = 0.460, p = 0.031). These findings suggest that DCE-MRI reflects tumour biology, providing prognostic information in patients with GC.
Ma and colleagues [17] compared DCE-MRI parameters in different histological subtypes of GC and investigated their correlation with vascular endothelial growth factor (VEGF) expression levels in 32 patients treated with surgical resection. Differently from the other studies, the ROIs were placed only on the lesions and the size was constant for each patient (10 mm). Mucinous adenocarcinomas showed higher Ve (0.491) and lower Ktrans (0.077 min−1) values than non-mucinous tumours (0.288 and 0.274 min−1, respectively) (p < 0.01). Differences were also observed for the Lauren classification, as the diffuse type showed higher Ve and Ktrans (0.466 and 0.249 min−1, respectively) values than the intestinal type (0.253 and 0.183 min−1, respectively) (p < 0.001). Additionally, Ktrans showed a significant correlation with the level of VEGF expression (ρ = 0.762, p < 0.001). Ktrans and VEGF are both related to the endothelial and microvascular permeability, which are in turn related to the neo-angiogenesis that is seen in tumours: in other words, a higher Ktrans is related to a higher level of VEGF, which is strictly related to a greater degree of angiogenesis. Together with the previous study [16], these findings suggest that angiogenesis increases the extravasation of gadolinium from the intravascular to the interstitial space, supporting the role of DCE-MRI as a potential tool to differentiate GC according to different histopathological features.
Li and colleagues [18] compared the performance of conventional breath-hold to free-breathing DCE-MRI using volume-interpolated breath-hold examination sequences. DCE-MRI parameters of normal gastric wall and GC were collected and perfusion parameters for both normal and pathological gastric walls were obtained. Kep was lower (0.750 vs 1.081 min−1; p < 0.05) while Ve was higher in GC (0.228 vs 0.162; p < 0.05). No significant differences for Ktrans and iAUC values between normal and pathological gastric walls were observed (p > 0.05).
Some examples of DCE-MRI in GC are shown in Figs. 2, 3 and 4.
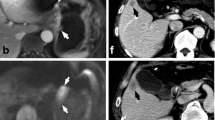
DCE-MRI showing a tumour of the gastric antrum (a) in a 73-year-old male. The Ktrans (b) was 0.279 min−1, the Kep (c) was 0.605 min−1 and the Ve (d) was 0.482. Final pathology (e): diffuse type (Lauren classification), staged as pT4aN3. DCE-MRI of a tumour of the gastro-oesophageal junction (Siewert III) (f) in a 68-year-old male. The Ktrans (g) was 0.117 min−1, the Kep (h) was 0.461 min−1 and the Ve (i) was 0.253. Final pathology (j): mixed type (Lauren classification), staged as pT3N1. DCE-MRI of a tumour of the gastric antrum (k) in a 49-year-old male. The Ktrans (l) was 0.016 min−1, the Kep (m) was 0.575 min−1 and the Ve (n) was 0.029. Final pathology (o): intestinal type (Lauren classification), staged as pT4aN2
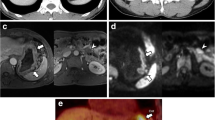
DCE-MRI showing a tumour of the gastric antrum (a) in a 66-year-old female. In the pretreatment scan, the Ktrans (b) was 0.078 min−1, the Kep (c) was 0.237 min−1 and the Ve (d) was 0.347. The tumour was confirmed at biopsy (e). In the posttreatment scan, there was a reduction in tumour size (f), and the Ktrans (g) was 0.070 min−1, the Kep (h) was 0.295 min−1 and the Ve (i) was 0.263. Final pathology (j): intestinal type (Lauren classification), staged as ypT1bN0 (tumour regression grade 1)
DCE-MRI of a tumour of the gastric antrum (a) in a 61-year-old female. In the pretreatment scan, the Ktrans (b) was 0.085 min−1, the Kep (c) was 0.176 min−1 and the Ve (d) was 0.539. The tumour was confirmed at biopsy (e). In the posttreatment scan, the tumour is still visible (f), and the Ktrans (g) was 0.128 min−1, the Kep (h) was 0.297 min−1 and the Ve (i) was 0.455. Final pathology (j): diffuse type (Lauren classification), staged as ypT3N0 (tumour regression grade 3)
18F-FDG PET/CT and image-derived quantitative parameters
18F-FDG PET/CT is recommended for patients with newly diagnosed GC if clinically indicated and if metastatic cancer is not evident, as well as in the posttreatment assessment and restaging.
The standardised uptake value (SUV) from 18F-FDG PET/CT is a dimensionless ratio used to distinguish between normal and abnormal levels of glucose uptake and can be considered an image-derived semi-quantitative parameter, defined as the ratio activity per unit volume of a ROI to the activity per unit whole-body volume (Figs. 5 and 6) [19].
18F-FDG PET/CT scan of a 72-year-old man with gastro-oesophageal junction cancer (a–d) demonstrated by an intense uptake of 18F-FDG before treatment (SUVmax = 10.7) (c). After two cycles of chemotherapy (paclitaxel + cisplatin + fluorouracil) (e–h), the SUVmax of the lesion decreased to 4.8 (g), showing good response to the therapy. Final pathology (i) ypT3N0 (tumour regression grade 1)
18F-FDG PET/CT scan of a 48-year-old woman with gastric cancer (a–d) demonstrated by an intense uptake of 18F-FDG before treatment (SUVmax = 4.7) (c). After one cycle of chemotherapy (capecitabine + paclitaxel) (e–h), no significant changes in 18F-FDG uptake (SUVmax = 4.8) were observed (g). Final pathology (i) ypT4aN1 (tumour regression grade 3)
18F-FDG PET/CT to assess the primary lesion in gastric cancer
Table 2 summarises the studies on the role of 18F-FDG PET/CT to assess the primary lesion in GC.
Stahl and colleagues [20] analysed the relationship between SUVmean and different tumour features from biopsy (including intestinal vs non-intestinal) in 40 patients. PET had a sensitivity of 60% in identifying locally advanced GC and the SUVmean was higher in the intestinal than in the non-intestinal type (6.7 vs 4.8; p = 0.03). No significant differences in the survival rate of patients with or without FDG accumulation (SUVmean cut-off, 4.6; p = 0.75) were observed. A clear limitation of this study is that the reference standard was biopsy and not radical surgery.
Mochiki and colleagues [21] reported a significant association between SUVmean and the depth of invasion, tumour size and nodal metastasis. They compared 18F-FDG PET findings with CT and found that 18F-FDG PET was less accurate for nodal staging (23% vs 65%). The SUVmean was higher for T2–T4 than T1 tumours (p < 0.05). Differently from the previous study [20], they observed a significant difference in the survival rate (p < 0.05).
Chen and colleagues [22] reported a sensitivity of 94% for 18F-FDG PET/CT (SUVmean = 7) and a significant association between FDG uptake and tumour size, nodal involvement and other histological features. They were among the first showing that the combination of 18F-FDG PET and CT was more accurate for preoperative staging than either modality alone (66% vs 51%, 66% vs. 47%; p = 0.002).
Oh and colleagues [23] performed a retrospective 18F-FDG PET/CT analysis of 136 patients treated with radical surgery. They set a threshold for SUVpeak from primary tumour of 3.2 to define hypermetabolic lesions and found that this was associated with tumour depth and nodal involvement (p < 0.001). The sensitivity and specificity for nodal involvement using the aforementioned threshold were 75% and 74% respectively.
Another group [24] reported the relationship between measurable and non-measurable GC on 18F-FDG PET/CT (defined as 1.35*SUVmax of liver+2*standard deviation of liver SUV). Among different parameters, a higher proportion of measurable tumours was found in well- or moderately differentiated GC than poorly differentiated tumours (71% vs 33% p < 0.05). Differently from the previous study [24], there was no difference for primary tumour stage and nodal metastasis.
Namikawa and colleagues [25] reported a sensitivity of 79% for the detection of GC for 18F-FDG PET/CT and a significant difference for SUVmax for patients with T3/T4 vs T1/T2 (9.0 vs. 3.8; p < 0.001), with and without distant metastasis (9.5 vs. 7.7; p = 0.018), and between stage III/IV and stage I/II (9.0 vs. 4.7; p = 0.017) after radical surgery. The SUVmax of the primary tumour was correlated with tumour size (r = 0.461; p < 0.001). The sensitivity, specificity and accuracy of 18F-FDG PET/CT for nodal involvement were 64%, 86% and 71% respectively.
18F-FDG PET/CT in treatment response of gastric cancer
We found six studies reporting on 18F-FDG PET/CT and treatment response in GC (Table 3).
Stahl and colleagues [26] compared different 18F-FDG PET/CT protocols and calculations of the SUVmean (time delay after 18F-FDG administration, acquisition protocol, reconstruction algorithm, SUV normalisation) for the early prediction of treatment response at baseline and after the first cycle of chemotherapy. They did not find any significant difference in the baseline and follow-up SUVmean calculation between protocols (p > 0.05), but higher SUV changes for responders than non-responders were observed (p < 0.01). They were among the first to demonstrate the robustness of 18F-FDG PET/CT for therapeutic monitoring, supporting the comparability of studies obtained with different protocols.
Vallböhmer and colleagues [27] analysed the differences in pre- and posttreatment SUVmax between responders and non-responders using the same histological definition as Stahl [26] (i.e. < 10% viable tumour cells in the specimen) but no correlation with treatment response was observed (p = 0.733). Significant differences in SUVmax were observed for the Lauren classification (p = 0.023) and tumour location (p = 0.041).
In another study on 17 patients [28] undergoing diffusion-weighted MRI and 18F-FDG PET/CT before and after treatment, no differences in treatment response were observed for pre- or posttreatment SUVmean (and their percentage change) (p = 0.605, p = 0.524 and p = 0.480). Treatment response was based on tumour regression grade (TRG) [32] and responders were considered TRG 1, 2 and 3 (i.e. including patients with more than 10% of viable cells).
Two studies [29, 30] evaluated the relationship between SUVmax and treatment response in advanced GC (i.e. no surgical specimens were used as the reference standard). Although follow-up imaging was performed at different time points (14 days vs 6 weeks after the start of chemotherapy) and different SUV thresholds for response were applied (40% vs 50%), both studies showed that metabolic changes in 18F-FDG PET/CT are predictive markers for response disease also for advanced GC. One study [30] showed a correlation between human epidermal growth factor HER2 status positivity (i.e. more aggressive cancer) and higher SUV uptake (p = 0.002).
Schneider and colleagues [31] reported that 18F-FDG PET/CT is able to detect non-responders (sensitivity, 91%; specificity, 47%; positive predictive value, 50%; negative predictive value, 90%; accuracy, 63%) but they could not prove that 18F-FDG PET/CT after the first cycle of chemotherapy can predict overall pathological response.
Similarly to the PRIDE study in oesophageal cancer [33], there is growing interest to develop models that predict the probability of response to neoadjuvant therapy in GC based on quantitative parameters derived from MRI and 18F-FDG PET/CT. However, given the controversial results at this regard [34], further studies are needed.
18F-FDG PET/CT in the prognosis of gastric cancer
We found eight studies on 18F-FDG PET/CT and prognosis in GC (Table 4). Significant results on the relationship between SUVmax and SUVmean and overall survival were reported by seven of them [35,36,37,38, 40,41,42], even though each study used different SUVmax and SUV mean cut-offs (Table 4). The study that did not show any significant difference in SUVmax and SUVmean with regard to prognosis was performed by Grabinska and colleagues [39]. A possible explanation is that a long range of follow-up was introduced in this study (range, 6 days to 5.2 years; median, 9.5 months), as also reported by the same authors. Therefore, the survival analysis from their study should be interpreted with caution. However, there is evidence of the relationship between SUVmax and SUVmean and prognosis in GC (Table 4).
18F-FDG PET/CT and radiomics in gastric cancer
There is growing evidence of the importance of radiomics in medical imaging [43] and this applies also to 18F-FDG PET/CT findings [44, 45].
A recent review has shown the promising role of radiomics obtained from different techniques—including 18F-FDG PET/CT—in gastro-oesophageal tumours [46].
Jiang and colleagues [47] have also developed a dedicated radiomic score using the features from 18F-FDG PET/CT in GC. In their study, they concluded that the radiomic signature was a powerful predictor of overall and disease-free survival and could add prognostic value to the traditional staging system.
However, as the current literature on this specific topic is still preliminary, there is a need of standardisation and different multicentre studies before including radiomics from 18F-FDG PET/CT in the clinical routine for GC.
Limitations
Quantitative imaging is becoming an increasingly common tool in modern radiology and its potential impact on patient care and on clinical outcomes is huge. However, it is broadly accepted that surrogate quantitative parameters of tumour biology assessed by imaging still require extensive standardisation and validation to proof that the surrogate represents the pathophysiological process under investigation. As reported by Rosenkrantz and colleagues [48], there are some practical aspects that should be considered when discussing the role of image-derived quantitative parameters. These are (i) accuracy (of a measurement, for example); (ii) repeatability and (iii) reproducibility (especially when quantitative imaging is performed in serial scans over time, as this allows to discriminate measurement error from biologic change) and (iv) clinical validity (i.e. impacting and improving patient’s life).
Therefore, some limitations from the papers discussed in this study should be reported. Firstly, for DCE-MRI, our review shows that the ROIs in all studies have been drawn on one selected axial section. This represents an important limitation, as these findings may be less representative of the whole tumour. Future studies should perform quantitative analysis on the whole volume obtained by contouring the tumour borders on each slice by planimetry. There is also a lack of optimised perfusion MRI protocols, dedicated postprocessing software programmes and high variability between MR scanners.
As far as 18F-FDG PET/CT imaging is concerned, a clear limitation is that the SUV is dependent on many factors including the ROI delineation, the activity injected, plasma glucose levels, and body size. There is variability between 18F-FDG PET/CT scanners, as well as in the accuracy of the image reconstruction and correction algorithms. The increased 18F-FDG uptake can be also seen in inflammatory or granulomatous processes and in sites of physiological tracer biodistribution.
Gastric distention, achieved by the consumption of water, milk or foaming agents before scanning, and a late-time-point 18F-FDG PET/CT scanning can relatively differentiate the physiological uptake from the malignant lesion.
Finally, standardised guidelines on how to interpret the quantitative results from DCE-MRI and 18F-FDG PET/CT have yet to be reported.
Conclusions
Similarly to the ADC from diffusion-weighted MRI and texture analysis from CT [2], different image-derived quantitative parameters from DCE-MRI and 18F-FDG PET/CT are promising tools in the management of GC. However, extensive standardisation and validation are still required before they can become an essential cornerstone for GC.
Abbreviations
- 18F-FDG PET/CT:
-
18F-Fluorodeoxyglucose positron emission tomography/computed tomography
- ADC:
-
Apparent diffusion coefficient
- CT:
-
Computed tomography
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- EGFR:
-
Epidermal growth factor receptor
- GC:
-
Gastric cancer
- SUV:
-
Standardised uptake value
- VEGF:
-
Vascular endothelial growth factor
- HER:
-
Human epidermal growth factor
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Giganti F, Tang L, Baba H (2019) Gastric cancer and imaging biomarkers: Part 1 - a critical review of DW-MRI and CE-MDCT findings. Eur Radiol 29(4):1743–1753. https://doi.org/10.1007/s00330-018-5732-4
Giganti F, Orsenigo E, Arcidiacono PG et al (2016) Preoperative locoregional staging of gastric cancer: is there a place for magnetic resonance imaging? Prospective comparison with EUS and multidetector computed tomography. Gastric Cancer 19(1):216–225
Richman DM, Tirumani SH, Hornick JL et al (2017) Beyond gastric adenocarcinoma: multimodality assessment of common and uncommon gastric neoplasms. Abdom Radiol (NY) 42(1):124–140
Brenkman HJF, Gertsen EC, Vegt E et al (2018) Evaluation of PET and laparoscopy in STagIng advanced gastric cancer: a multicenter prospective study (PLASTIC-study). BMC Cancer 18(1):450
European Society of Radiology (ESR) (2010) White paper on imaging biomarkers. Insights Imaging 1(2):42–45
Buckler AJ, Bresolin L, Dunnick NR, Sullivan DC (2011) A collaborative enterprise for multi-stakeholder participation in the advancement of quantitative imaging. Radiology 258(3):906–914
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 7(1):91–101
O’Connor JP, Tofts PS, Miles KA, Parkes LM, Thompson G, Jackson A (2011) Dynamic contrast-enhanced imaging techniques: CT and MRI. Br J Radiol 84(special_issue_2):S112–S120
Kershaw LE, Cheng HLM (2010) Temporal resolution and SNR requirements for accurate DCE-MRI data analysis using the AATH model. Magn Reson Med 64(6):1772–1780
Nishida N, Yano H, Nishida T, Kamura T, Kojiro M (2006) Angiogenesis in cancer. Vasc Health Risk Manag 2(3):213–219
Tonini T, Rossi F, Claudio PP (2003) Molecular basis of angiogenesis and cancer. Oncogene 22(42):6549–6556
Cuenod CA, Balvay D (2013) Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging 94(12):1187–1204
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Kang BC, Kim JH, Kim KW et al (2000) Abdominal imaging value of the dynamic and delayed MR sequence with Gd-DTPA in the T-staging of stomach cancer: correlation with the histopathology. Abdom Imaging 25:14–24
Joo I, Lee JM, Han JK, Yang HK, Lee HJ, Choi BI (2015) Dynamic contrast-enhanced MRI of gastric cancer: correlation of the perfusion parameters with pathological prognostic factors. J Magn Reson Imaging 41(6):1608–1614
Ma L, Xu X, Zhang M et al (2017) Dynamic contrast-enhanced MRI of gastric cancer: correlations of the pharmacokinetic parameters with histological type, Lauren classification, and angiogenesis. Magn Reson Imaging 37:27–32
Li HH, Zhu H, Yue L et al (2018) Feasibility of free-breathing dynamic contrast-enhanced MRI of gastric cancer using a golden-angle radial stack-of-stars VIBE sequence: comparison with the conventional contrast-enhanced breath-hold 3D VIBE sequence. Eur Radiol 28(5):1891–1899
Thie JA (2004) Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med 45(9):1431–1434
Stahl A, Ott K, Weber WA et al (2003) FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 30(2):288–295
Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K (2004) Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg 28(3):247–253
Chen J, Cheong JH, Yun MJ et al (2005) Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 103(11):2383–2390
Oh HH, Lee SE, Choi IS et al (2011) The peak-standardized uptake value (P-SUV) by preoperative positron emission tomography-computed tomography (PET-CT) is a useful indicator of lymph node metastasis in gastric cancer. J Surg Oncol 104(5):530–533
Oh SY, Cheon GJ, Kim YC, Jeong E, Kim S, Choe JG (2012) Detectability of T-measurable diseases in advanced gastric cancer on FDG PET-CT. Nucl Med Mol Imaging 46(4):261–268
Namikawa T, Okabayshi T, Nogami M, Ogawa Y, Kobayashi M, Hanazaki K (2014) Assessment of 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography in the preoperative management of patients with gastric cancer. Int J Clin Oncol 19(4):649–655
Stahl A, Ott K, Schwaiger M, Weber WA (2004) Comparison of different SUV-based methods for monitoring cytotoxic therapy with FDG PET. Eur J Nucl Med Mol Imaging 31(11):1471–1479
Vallböhmer D, Hölscher AH, Schneider PM et al (2010) [18F]-Fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemotherapy in gastric cancer. J Surg Oncol 102(2):135–140
Giganti F, De Cobelli F, Canevari C et al (2014) Response to chemotherapy in gastric adenocarcinoma with diffusion-weighted MRI and 18 F-FDG-PET/CT: correlation of apparent diffusion coefficient and partial volume corrected standardized uptake value with histological tumor regression grade. J Magn Reson Imaging 40(5):1147–1157
Wang C, Guo W, Zhou M et al (2016) The predictive and prognostic value of early metabolic response assessed by positron emission tomography in advanced gastric cancer treated with chemotherapy. Clin Cancer Res 22(7):1603–1610
Park S, Ha S, Kwon HW et al (2017) Prospective evaluation of changes in tumor size and tumor metabolism in patients with advanced gastric cancer undergoing chemotherapy: association and clinical implication. J Nucl Med 58(6):899–904
Schneider PM, Eshmuminov D, Rordorf T et al (2018) 18FDG-PET-CT identifies histopathological non-responders after neoadjuvant chemotherapy in locally advanced gastric and cardia cancer: cohort study. BMC Cancer 18:548
Mandard AM, Dalibard F, Mandard JC et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: clinicopathologic correlations. Cancer 73(11):2680–2686
Borggreve AS, Mook S, Verheij M et al (2018) Preoperative image-guided identification of response to neoadjuvant chemoradiotherapy in esophageal cancer (PRIDE): a multicenter observational study. BMC Cancer 18(1):1006
Kwee RM, Kwee TC (2014) Role of imaging in predicting response to neoadjuvant chemotherapy in gastric cancer. World J Gastroenterol 20(7):1650–1656
Pak KH, Yun M, Cheong JH, Hyung WJ, Choi SH, Noh SH (2011) Clinical implication of FDG-PET in advanced gastric cancer with signet ring cell histology. J Surg Oncol 104(6):566–570
Park JC, Lee J-H, Cheoi K et al (2012) Predictive value of pretreatment metabolic activity measured by fluorodeoxyglucose positron emission tomography in patients with metastatic advanced gastric cancer: the maximal SUV of the stomach is a prognostic factor. Eur J Nucl Med Mol Imaging 39(7):1107–1116
Lee JW, Lee SM, Lee M-S, Shin HC (2012) Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging 39(9):1425–1434
Kim J, Lim ST, Na CJ et al (2014) Pretreatment F-18 FDG PET/CT parameters to evaluate progression-free survival in gastric cancer. Nucl Med Mol Imaging 48(1):33–40
Grabinska K, Pelak M, Wydmanski J, Tukiendorf A, d’Amico A (2015) Prognostic value and clinical correlations of 18-fluorodeoxyglucose metabolism quantifiers in gastric cancer. World J Gastroenterol 21(19):5901–5909
Na SJ, o JH, Park JM et al (2016) Prognostic value of metabolic parameters on preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with stage III gastric cancer. Oncotarget 7(39)
Lee S, Seo HJ, Kim S, Eo JS, Oh SC (2017) Prognostic significance of interim 18 F-fluorodeoxyglucose positron emission tomography-computed tomography volumetric parameters in metastatic or recurrent gastric cancer. Asia Pac J Clin Oncol:1–8
Chon HJ, Kim C, Cho A et al (2018) The clinical implications of FDG-PET/CT differ according to histology in advanced gastric cancer. Gastric Cancer 22(1):113–122. https://doi.org/10.1007/s10120-018-0847-5
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577
Cook GJR, Azad G, Owczarczyk K, Siddique M, Goh V (2018) Challenges and promises of PET radiomics. Int J Radiat Oncol Biol Phys 102(4):1083–1089
Lovinfosse P, Visvikis D, Hustinx R, Hatt M (2018) FDG PET radiomics: a review of the methodological aspects. Clin Transl Imaging 6:379–391
Sah BR, Owczarczyk K, Siddique M, Cook GJR, Goh V (2018) Radiomics in esophageal and gastric cancer. Abdom Radiol (NY) 44(6):2048–2058. https://doi.org/10.1007/s00261-018-1724-1728
Jiang Y, Yuan Q, Lv W et al (2018) Radiomic signature of 18F fluorodeoxyglucose PET/CT for prediction of gastric cancer survival and chemotherapeutic benefits. Theranostics 8(21):5915–5928
Rosenkrantz AB, Mendiratta-Lala M, Bartholmai BJ et al (2015) Clinical utility of quantitative imaging. Acad Radiol 22(1):33–49
Funding
Francesco Giganti is funded by the UCL Graduate Research Scholarship and the Brahm PhD scholarship in memory of Chris Adams. Lei Tang is funded by National Key R&D Program of China (No. 2018YFC0910700) and Beijing Natural Science Foundation (No. Z180001)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Francesco Giganti.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study.
Ethical approval
Institutional Review Board approval was not required.
Methodology
• Review
• Multicentre study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tang, L., Wang, XJ., Baba, H. et al. Gastric cancer and image-derived quantitative parameters: Part 2—a critical review of DCE-MRI and 18F-FDG PET/CT findings. Eur Radiol 30, 247–260 (2020). https://doi.org/10.1007/s00330-019-06370-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06370-x