Abstract
Objectives
To intraindividually compare the signal-enhancing effect of 0.5 M gadoterate meglumine and 1.0 M gadobutrol in dynamic contrast-enhanced magnetic resonance (DCE-MR) imaging of the prostate.
Methods
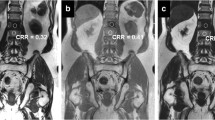
Fifty patients who underwent two 3-T MR examinations of the prostate were included in this IRB-approved retrospective uncontrolled, unrandomized study. All received two scans (mean time interval, 20.5 months) including T1-weighted DCE-MR imaging, one with 0.5 M gadoterate meglumine and one with 1.0 M gadobutrol. Equimolar doses of gadolinium (0.1 mmol/kg body weight) were administered with identical injection speed (2 mL/s), resulting in differing gadolinium delivery rate. An identical region of interest (ROItz) within a BPH-node was identified on both scans. The area under the time-enhancement curve of each ROItz from 0 to 180 s post contrast arrival and pharmacokinetic parameters were calculated. Relative enhancement and signal-to-noise (SNR) and contrast-to-noise (CNR) ratios in the delayed phase at about 180 s were compared between both agents.
Results
There was a significantly larger area under the time-enhancement curve (5.53 vs 4.97 p = 0.0007) and higher relative enhancement of BPH nodules (2.23 vs 1.96 p < 0.0001) with gadobutrol compared with gadoterate meglumine. There were no significant differences in SNR (44.55 vs 37.63 p = 0.12), CNR (31.22 vs 26.39 p = 0.18), and pharmacokinetic parameters Ktrans (0.31 vs 0.32 p = 0.86), Ve (1.36 vs 0.98 p = 0.13), and Kep (0.34 vs 0.36 p = 0.12).
Conclusions
At equimolar doses, increased gadolinium delivery over time using gadobutrol provides higher relative enhancement parameters in BPH nodules compared with gadoterate meglumine, but does not translate into improved SNR or CNR.
Key Points
• At equal injection rate and equimolar total dose, gadobutrol compared with gadoterate meglumine provides a significantly greater relative enhancement in DCE-MR imaging of BPH over the first 180 s.
• There are no significant differences in SNRs, CNRs, and pharmacokinetic parameters between the two GBCAs.




Similar content being viewed by others
Abbreviations
- AUCenh :
-
Area under the RE-t curve from contrast agent arrival time to 180 s after arrival
- BPH:
-
Benign prostatic hyperplasia
- CNR:
-
Contrast-to-noise ratio
- DCE-MR:
-
Dynamic contrast-enhanced magnetic resonance
- GBCA:
-
Gadolinium-based contrast agent
- GRAPPA:
-
GeneRalized Autocalibrating Partial Parallel Acquisition
- IRB:
-
Institutional Review Board
- Kep:
-
Flux rate constant
- Ktrans:
-
Volume transfer constant reflecting the efflux rate of gadolinium contrast from blood plasma into the interstitial space
- MR:
-
Magnetic resonance
- RE:
-
Relative enhancement
- RE-t:
-
Relative enhancement over time
- ROItz :
-
Region of interest corresponding to a BPH nodule in the transition zone
- SNR:
-
Signal-to-noise ratio
- t 180 :
-
Time point closest to 180 s after first DCE-MR acquisition
- TE:
-
Echo time
- TR:
-
Repetition time
- Ve:
-
Extravascular extracellular space corresponding to the interstitial space
References
Bellin MF, Van Der Molen AJ (2008) Extracellular gadolinium-based contrast media: an overview. Eur J Radiol 66:160–167
Rohrer M, Bauer H, Mintorovitch J, Reguardt M, Weinmann HJ (2005) Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 40:715–724
Cuenod CA, Balvay D (2013) Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging 94:1187–1204
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 7:91–101
Attenberger UI, Runge VM, Morelli JN, Williams J, Jackson CB, Michaely HJ (2010) Evaluation of gadobutrol, a macrocyclic, nonionic gadolinium chelate in a brain glioma model: comparison with gadoterate meglumine and gadopentetate dimeglumine at 1.5 T, combined with an assessment of field strength dependence, specifically 1.5 versus 3 T. J Magn Reson Imaging 31:549–555
Fallenberg EM, Renz DM, Karle B et al (2015) Intraindividual, randomized comparison of the macrocyclic contrast agents gadobutrol and gadoterate meglumine in breast magnetic resonance imaging. Eur Radiol 25:837–849
Durmus T, Vollnberg B, Schwenke C et al (2013) Dynamic contrast enhanced MRI of the prostate: comparison of gadobutrol and Gd-DTPA. Rofo 85:862–868
Kulh CK, Mielcarek P, Klaschik S et al (1999) Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 211:101–110
Tofts PS, Kermode AG (1991) Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO (2007) Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging 26:375–385
Motulsky HJ, Ransnas LA (1987) Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J 1:365–374
Prince MR, Lee HG, Lee CH et al (2017) Safety of gadobutrol in over 23,000 patients: the GARDIAN study, a global multicentre, prospective, non-interventional study. Eur Radiol 27:286–295
Elster AD (1997) How much contrast is enough? Dependence of enhancement on field strength and MR pulse sequence. Eur Radiol 7:S276–S280
Anzalone N, Scarabino T, Venturi C et al (2013) Cerebral neoplastic enhancing lesions: multicenter, randomized, crossover intraindividual comparison between gadobutrol (1.0M) and gadoterate meglumine (0.5M) at 0.1 mmol Gd/kg body weight in a clinical setting. Eur J Radiol 82:139–145
Maravilla KR, San-Juan D, Kim SJ et al (2017) Comparison of gadoterate meglumine and gadobutrol in the MRI diagnosis of primary brain tumors: a double-blind randomized controlled intraindividual crossover study (the REMIND study). AJNR Am J Neuroradiol 38:1681–1688
Saake M, Langner S, Schwenke C et al (2016) MRI in multiple sclerosis: an intra-individual, randomized and multicentric comparison of gadobutrol with gadoterate meglumine at 3 T. Eur Radiol 26:820–828
Lancelot E, Froehlich J, Heine O, Desché P (2016) Effects of gadolinium-based contrast agent concentrations (0.5 M or 1.0 M) on the diagnostic performance of magnetic resonance imaging examinations: systematic review of the literature. Acta Radiol 57:1334–1343
Haneder S, Attenberger UI, Schoenberg SO, Loewe C, Arnaiz J, Michaely HJ (2012) Comparison of 0.5 M gadoterate and 1.0 M gadobutrol in peripheral MRA: a prospective, single-center, randomized, crossover, double-blind study. J Magn Reson Imaging 36:1213–1221
Szucs-Farkas Z, Froehlich JM, Ulrich M et al (2008) 1.0-M gadobutrol versus 0.5-M gadoterate for peripheral magnetic resonance angiography: a prospective randomized controlled clinical trial. J Magn Reson Imaging 27:1399–1405
Kramer JH, Arnoldi E, François CJ et al (2013) Dynamic and static magnetic resonance angiography of the supra-aortic vessels at 3.0 T: intraindividual comparison of gadobutrol, gadobenate dimeglumine, and gadoterate meglumine at equimolar dose. Invest Radiol 48:121–128
Hoelter P, Lang S, Weibart M et al (2017) Prospective intraindividual comparison of gadoterate and gadobutrol for cervical and intracranial contrast-enhanced magnetic resonance angiography. Neuroradiology 59:1233–1239
Loewe C, Arnaiz J, Krause D, Marti-Bonmati L, Haneder S, Kramer U (2015) MR angiography at 3 T of peripheral arterial disease: a randomized prospective comparison of gadoterate meglumine and gadobutrol. AJR Am J Roentgenol 204:1311–1321
Renz DM, Durmus T, Böttcher J et al (2014) Comparison of gadoteric acid and gadobutrol for detection as well as morphologic and dynamic characterization of lesions on breast dynamic contrast-enhanced magnetic resonance imaging. Invest Radiol 49:474–484
Pediconi F, Catalano C, Padula S et al (2008) Contrast-enhanced MR mammography: improved lesion detection and differentiation with gadobenate dimeglumine. AJR Am J Roentgenol 191:1339–1346
Martincich L, Faivre-Pierret M, Zechmann CM et al (2011) Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for breast MR imaging (DETECT trial). Radiology 258:396–408
Franiel T, Hamm B, Hricak H (2011) Dynamic contrast-enhanced magnetic resonance imaging and pharmacokinetic models in prostate cancer. Eur Radiol 21:616–626
De Visschere PJL, Vral A, Perletti G et al (2017) Multiparametric magnetic resonance imaging characteristics of normal, benign and malignant conditions in the prostate. Eur Radiol 27:2095–2109
Brown DL, Lalla CD, Masselink AJ (2013) AUC versus peak-trough dosing of vancomycin: applying new pharmacokinetic paradigms to an old drug. Ther Drug Monit 35:443–449
Tombach B, Benner T, Reimer P et al (2003) Do highly concentrated gadolinium chelates improve MR brain perfusion imaging? Intraindividually controlled randomized crossover concentration comparison study of 0.5 versus 1.0 mol/L gadobutrol. Radiology 226:880–888
Weishaupt D, Köchli VD, Marincek B (2003) Factors affecting the signal-to-noise ratio. In: How does MRI work? Springer, Berlin, pp 31–42
Kim YK, Lee YH, Kim CS, Han YM, Hwang SB (2008) Double-dose 1.0-M gadobutrol versus standard-dose 0.5-M gadopentetate dimeglumine in revealing small hypervascular hepatocellular carcinomas. Eur Radiol 18:70–77
Pennekamp W, Roggenland D, Hering S et al (2011) Intraindividual, randomised comparison of the MRI contrast agents gadobutrol and gadoterate in imaging the distal lower limb of patients with known or suspected osteomyelitis, evaluated in an off-site blinded read. Eur Radiol 21:1058–1067
Escribano F, Sentís M, Oliva JC et al (2018) Dynamic magnetic resonance imaging of the breast: comparison of gadobutrol vs. Gd-DTPA. Radiologia 60:49–56
Frenzel T, Lengsfeld P, Schirmer H, Hütter J, Weinmann HJ (2008) Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol 43:817–828
Gillis A, Gray M, Burstein D (2002) Relaxivity and diffusion of gadolinium agents in cartilage. Magn Reson Med 48:1068–1071
Wiener E, Woertler K, Weirich G, Rummeny EJ, Settles M (2007) Contrast enhanced cartilage imaging: comparison of ionic and non-ionic contrast agents. Eur J Radiol 63:110–119
Mathur SK, Gupta S, Marwah N, Narula A, Singh S, Arora B (2003) Significance of mucin stain in differentiating benign and malignant lesions of prostate. Indian J Pathol Microbiol 46:593–595
Khanna A, Patil R, Deshmukh A (2014) Assessment of the potential of pathological stains in human prostate cancer. J Clin Diagn Res 8:124–128
Chesnais AL, Niaf E, Bratan F et al (2013) Differentiation of transitional zone prostate cancer from benign hyperplasia nodules: evaluation of discriminant criteria at multiparametric MRI. Clin Radiol 68:e323–e330
van Niekerk CG, Witjes JA, Barentsz JO, van der Laak JA, Hulsbergen-van de Kaa CA (2013) Microvascularity in transition zone prostate tumors resembles normal prostatic tissue. Prostate 73:467–475
Lemaitre L, Puech P, Poncelet E et al (2009) Dynamic contrast-enhanced MRI of anterior prostate cancer: morphometric assessment and correlation with radical prostatectomy findings. Eur Radiol 19:470–480
Purysko AS, Rosenkrantz AB, Barentsz JO, Weinreb JC, Macura KJ (2016) PI-RADS version 2: a pictorial update. Radiographics 36:1354–1372
Drew PJ, Chatterjee S, Turnbull LW et al (1999) Dynamic contrast enhanced magnetic resonance imaging of the breast is superior to triple assessment for the pre-operative detection of multifocal breast cancer. Ann Surg Oncol 6:599–603
Pickles MD, Lowry M, Manton DJ, Turnbull LW (2015) Prognostic value of DCE-MRI in breast cancer patients undergoing neoadjuvant chemotherapy: a comparison with traditional survival indicators. Eur Radiol 25:1097–1106
Acknowledgements
The authors would like to thank Ms. Bettina Herwig for language editing of the manuscript.
Funding
The authors state that this work was partially-funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB 1340/1 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Patrick Asbach.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, C.H., Vellayappan, B., Taupitz, M. et al. Dynamic contrast-enhanced MR imaging of the prostate: intraindividual comparison of gadoterate meglumine and gadobutrol. Eur Radiol 29, 6982–6990 (2019). https://doi.org/10.1007/s00330-019-06321-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06321-6