Abstract
Objectives
To identify dynamic contrast-enhanced (DCE) imaging parameters from MRI, CT and US that are prognostic and predictive in patients with metastatic renal cell cancer (mRCC) receiving sunitinib.
Methods
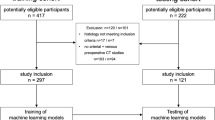
Thirty-four patients were monitored by DCE imaging on day 0 and 14 of the first course of sunitinib treatment. Additional scans were performed with DCE-US only (day 7 or 28 and 2 weeks after the treatment break). Perfusion parameters that demonstrated a significant correlation (Spearman p < 0.05) with progression-free survival (PFS) and overall survival (OS) were investigated using Cox proportional hazard models/ratios (HR) and Kaplan-Meier survival analysis.
Results
A higher baseline and day 14 value for Ktrans (DCE-MRI) and a lower pre-treatment vascular heterogeneity (DCE-US) were significantly associated with a longer PFS (HR, 0.62, 0.37 and 5.5, respectively). A larger per cent decrease in blood volume on day 14 (DCE-US) predicted a longer OS (HR, 1.45). We did not find significant correlations between any of the DCE-CT parameters and PFS/OS, unless a cut-off analysis was used.
Conclusions
DCE-MRI, -CT and ultrasound produce complementary parameters that reflect the prognosis of patients receiving sunitinib for mRCC. Blood volume measured by DCE-US was the only parameter whose change during early anti-angiogenic therapy predicted for OS and PFS.
Key Points
• DCE-CT, -MRI and ultrasound are complementary modalities for monitoring anti-angiogenic therapy.
• The change in blood volume measured by DCE-US was predictive of OS/PFS.
• Baseline vascular heterogeneity by DCE-US has the strongest prognostic value for PFS.



Similar content being viewed by others
References
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Weber WA (2009) Assessing tumor response to therapy. J Nucl Med 50:1S–10S
Desar IME, Herpen CMLV, Laarhoven HWMV, Barentsz JO, Oyen WJG, Graaf WTAVD (2009) Beyond RECIST: Molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer Treat Rev 35:309–321
Sirous R, Henegan JC, Zhang X, Howard CM, Souza F, Smith AD (2016) Metastatic renal cell carcinoma imaging evaluation in the era of anti-angiogenic therapies. Abdom Radiol 41:1086–1099
Mancuso M, Davis R, Norberg S et al (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Investig 116:2610–2621
Desar IME, Van Herpen CML, Van Laarhoven HWM, Barentsz JO, Oyen WJG, Van Der Graaf WTA (2009) Beyond RECIST: Molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer Treat Rev 35:309–321
O'Connor J, Aboagye E, Adams J, Aerts H, Barrington S, Beer A (2017) Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 14:169–186
Lassau N, Vilgrain V, Taieb S et al (2012) Evaluation with DCE-US of antiangiogenic treatments in 539 patients allowing the selection of one surrogate marker correlated to overall survival. J Clin Oncol abstract 4618
Leen E, Averkiou M, Arditi M et al (2012) Dynamic contrast enhanced ultrasound assessment of the vascular effects of novel therapeutics in early stage trials. Eur Radiol 22:1442–1450
O'Connor JPB, Jackson A, Parker GJM, Roberts C, Jayson GC (2012) Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol 9:167–177
Prezzi D, Khan A, Goh V (2015) Perfusion CT imaging of treatment response in oncology. Eur J Radiol 84:2380–2385
Hudson JM, Karshafian R, Burns PN (2009) Quantification of flow using ultrasound and microbubbles: A disruption replenishment model based on physical principles. Ultrasound Med Biol 35:2007–2020
Katabathina VS, Lassau N, Pedrosa I, Ng CS, Prasad SR (2012) Evaluation of treatment response in patients with metastatic renal cell carcinoma: Role of state-of-the-art cross-sectional imaging. Curr Urol Rep 13:70–81
Lamuraglia M, Bridal SL, Santin M et al (2010) Clinical relevance of contrast-enhanced ultrasound in monitoring anti-angiogenic therapy of cancer: current status and perspectives. Crit Rev Oncol Hematol 73:202–212
Lee TY (2002) Functional CT: Physiological models. Trends Biotechnol 20:S3–S10
Nathan P, Vinayan A (2013) Imaging techniques as predictive and prognostic biomarkers in renal cell carcinoma. Ther Adv Med Oncol 5:119–131
O'Connor JPB, Jackson A, Parker GJM, Jayson GC (2007) DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer 96:189–195
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Ammari S, Thiam R, Cuenod CA et al (2014) Radiological evaluation of response to treatment: Application to metastatic renal cancers receiving anti-angiogenic treatment. Diagn Interv Imaging 95:527–539
Lassau N, Chami L, Benatsou B, Peronneau P, Roche A (2007) Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol Suppl 17:89–98
Yankeelov TE, Niermann KJ, Huamani J et al (2006) Correlation between estimates of tumor perfusion from microbubble contrast-enhanced sonography and dynamic contrast-enhanced magnetic resonance imaging. J Ultrasound Med 25:487–497
Kim E, Kim J, Maelandsmo GM, Johansen B, Moestue SA (2016) Anti-angiogenic therapy affects the relationship between tumor vascular structure and function: A correlation study between micro-computed tomography angiography and dynamic contrast enhanced MRI. Magn Reson Med 25:25
Heng DYC, Xie W, Regan MM et al (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol 27:5794–5799
Williams R, Hudson JM, Lloyd BA et al (2011) Dynamic microbubble contrast-enhanced us to measure tumor response to targeted therapy: A proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology 260:581–590
Hudson JM (2013) Lognormal Perfusion Model. http://www.mathworks.com/matlabcentral/fileexchange/40786-lognormal-perfusionmodel
Hudson JM, Williams R, Lloyd B et al (2011) Improved flow measurement using microbubble contrast agents and disruption-replenishment: Clinical application to tumour monitoring. Ultrasound Med Biol 37:1210–1221
Hudson JM, Williams R, Karshafian R et al (2013) Quantifying vascular heterogeneity using microbubble disruption-replenishment kinetics inpatients with renal cell cancer. Investig Radiol 49(2):116–123
Ebos JML, Mastri M, Hudson JM et al (2015) Effect of the timing of sunitinib administration on the predictive value of biomarkers in renal cell cancer (mRCC). J Clin Oncol 33:11096–11096
Bjarnason GA, Khalil B, Hudson JM et al (2014) Outcomes in patients with metastatic renal cell cancer treated with individualized sunitinib therapy: Correlation with dynamic microbubble ultrasound data and review of the literature. Urol Oncol 32:480–487
Hahn OM, Yang C, Medved M et al (2008) Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol 26:4572–4578
Flaherty K, Rosen M, Heitjan D et al (2008) Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther 7:496–501
Desar IME, ter Voert EGW, Hambrock T et al (2012) Functional MRI techniques demonstrate early vascular changes in renal cell cancer patients treated with sunitinib: a pilot study. Cancer Imaging 11:259–265
Sourbron SP, Buckley DL (2011) On the scope and interpretation of the Tofts models for DCE-MRI. Magn Reson Med 66:735–745
Han KS, Jung DC, Choi HJ et al (2010) Pretreatment assessment of tumor enhancement on contrast-enhanced computed tomography as a potential predictor of treatment outcome in metastatic renal cell carcinoma patients receiving antiangiogenic therapy. Cancer 116:2332–2342
Fournier L, Oudard S, Thiam R et al (2010) Metastatic renal carcinoma: Evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT1. Radiology 256:511–518
van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Haanen JB, Boven E (2010) Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer 102:803–809
Nathan P, Vinayan A, Stott D, Juttla J, Goh V (2010) CT response assessment combining reduction in both size and arterial phase density correlates with time to progression in metastatic renal cancer patients treated with targeted therapies. Cancer Biol Ther 9:15–19
Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K (2013) Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics 33:1323–1341
Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM (2010) Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol 194:1470–1478
Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA (2011) Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261:165–171
Lassau N, Koscielny S, Albiges L et al (2010) Metastatic renal cell carcinoma treated with sunitinib: Early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res 16:1216–1225
Wei K, Jayaweera A, Firoozan S, Linka A, Skyba D, Kaul S (1998) Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97:473–483
Bjarnason GA, Knox JJ, Kollmannsberger CK et al (2017) Phase II study of individualized sunitinib (SUN) as first-line therapy for metastatic renal cell cancer. J Clin Oncol 35:4514–4514
Stride E, Tang M-X, Eckersley RJ (2009) Physical phenomena affecting quantitative imaging of ultrasound contrast agents. Applied Acoustics 70:1352–1362
Funding
This study has received funding from the Terry Fox Programme of the National Cancer Institute of Canada, the Canadian Institutes of Health Research and an investigator-initiated grant to Georg A. Bjarnason from Pfizer Canada.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Georg Bjarnason.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
Georg A. Bjarnason: Pfizer Canada: grant support for this study, CME presentations and travel support to oncology meetings. The remaining authors have no further conflicts to disclose.
Statistics and biometry
Alex Kiss (author) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
A subset of the patient cohort included in this article has been reported in the following journal publications. The referenced articles were reports on technical protocol development (3), a new DCE-US parameter development (2) or describing using DCE-US vascular volume changes during and after sunitinib therapy to justify changes in sunitinib scheduling (1):
1. Bjarnason G A, Khalil B, Hudson JM, Williams R, Milot LM, Atri M, et al. Outcomes in patients with metastatic renal cell cancer treated with individualized sunitinib therapy: Correlation with dynamic microbubble ultrasound data and review of the literature. Urol Oncol. Elsevier; 2013;32(4):1–8.
2. Hudson JM, Williams R, Karshafian R, Milot L, Atri M, Burns PN, et al. Quantifying vascular heterogeneity using microbubble disruption-replenishment kinetics in patients with renal cell cancer. Invest Radiol [Internet]. 2014;49(2):116–23.
3. Williams R, Hudson JJM, Lloyd BBA, Sureshkumar AR, Lueck G, Bjarnason GA, et al. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology [Internet]. 2011 [cited 2012 Oct 12];260(2):581–90.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Hudson, J.M., Bailey, C., Atri, M. et al. The prognostic and predictive value of vascular response parameters measured by dynamic contrast-enhanced-CT, -MRI and -US in patients with metastatic renal cell carcinoma receiving sunitinib. Eur Radiol 28, 2281–2290 (2018). https://doi.org/10.1007/s00330-017-5220-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5220-2