Abstract
Objectives
Differences in results of baseline and subsequent annual repeat rounds provide important information for optimising the regimen of screening.
Methods
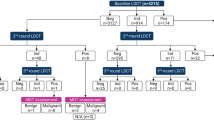
A prospective cohort study of 65,374 was reviewed to examine the frequency/percentages of the largest noncalcified nodule (NCN), lung cancer cell types and Kaplan–Meier (K-M) survival rates, separately for baseline and annual rounds.
Results
Of 65,374 baseline screenings, NCNs were identified in 28,279 (43.3%); lung cancer in 737 (1.1%). Of 74,482 annual repeat screenings, new NCNs were identified in 4959 (7%); lung cancer in 179 (0.24%). Only adenocarcinoma was diagnosed in subsolid NCNs. Percentages of lung cancers by cell type were significantly different (p < 0.0001) in the baseline round compared with annual rounds, reflecting length bias, as were the ratios, reflecting lead times. Long-term K-M survival rate was 100% for typical carcinoids and for adenocarcinomas manifesting as subsolid NCNs; 85% (95% CI 81–89%) for adenocarcinoma, 74% (95% CI 63–85%) for squamous cell, 48% (95% CI 34–62%) for small cell. The rank ordering by lead time was the same as the rank ordering by survival rates.
Conclusions
The significant differences in the frequency of NCNs and frequency and aggressiveness of diagnosed cancers in baseline and annual repeat need to be recognised for an optimal regimen of screening.
Key Points
• Lung cancer aggressiveness varies considerably by cell type and nodule consistency.
• Kaplan–Meier survival rates varied by cell type between 100% and 48%.
• The percentages of lung cancers by cell type in screening rounds reflect screening biases.
• Rank ordering by cell type survival is consistent with that by lead times.
• Empirical evidence provides critical information for the regimen of screening.

Similar content being viewed by others
Change history
13 February 2018
The original version of this article unfortunately contained a mistake. The conflict of interest was incorrect.
Abbreviations
- CT:
-
computed tomography
- ELCAP:
-
Early Lung Cancer Action Program
- I-ELCAP:
-
International Early Lung Cancer Action Program
- K-M:
-
Kaplan–Meier
- NCNs:
-
noncalcified nodules
- NLST:
-
National Lung Screening Trial
References
Hutchinson G, Shapiro S (1968) Lead time gained by diagnostic screening for breast cancer. J Natl Cancer Inst 41:665–681
Morrison AS (1982) The effects of early treatment, lead time and length bias on the mortality experienced by cases detected by screening. Int J Epidemiol 11:261–267
Miller AB, International Union against Cancer, UICC Project on Evaluation of Screening for Cancer (1991) Cancer screening: a report of the workshop to update conclusions on screening for cancer of sites previously considered and to evaluate some new sites, held at Selwyn College, Cambridge, UK, April 2–5, 1990. Cambridge University Press, New York xvi
Flehinger BJ, Kimmel M, Melamed MR (1992) The effect of surgical treatment on survival from early lung cancer. Implications Screen Chest 101:1013–1018
Morrison AS (1992) Screening in chronic disease, 2nd edn. Monographs in epidemiology and biostatistics. Oxford University Press, New York xiv
Bae JM (2014) Methodological issues for determining intervals of subsequent cancer screening. Epidemiol Health 36:e2014010
Draisma G, van Rosmalen J (2013) A note on the catch-up time method for estimating lead or sojourn time in prostate cancer screening. Stat Med 32:3332–3341
Prevost TC, Launoy G, Duffy SW, Chen HH (1998) Estimating sensitivity and sojourn time in screening for colorectal cancer: a comparison of statistical approaches. Am J Epidemiol 148:609–619
Henschke C, Yankelevitz D, Smith J, Miettinen O, ELCAP Group (2002) Screening for lung cancer: the early lung cancer action approach. Lung Cancer 35:143–148
International Early Lung Cancer Action Program (2016) I-ELCAP protocol documents http://www.IELCAP.org/protocols. Accessed 28 Aug 2017
Henschke C, Yankelevitz D, Mirtcheva R, McGuinness G, McCauley D, Miettinen O (2002) CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 178:1053–1057
Yankelevitz DF, Yip R, Smith JP et al (2015) CT screening for lung cancer: nonsolid nodules in baseline and annual repeat rounds. Radiology 277:555–564
Henschke CI, Yip R, Smith JP et al (2016) CT screening for lung cancer: part-solid nodules in baseline and annual repeat rounds. AJR Am J Roentgenol 207:1176–1184
Yip R, Yankelevitz DF, Hu M et al (2016) Lung cancer deaths in the National Lung Screening Trial attributed to nonsolid nodules. Radiology 281:589–596
Yip R, Yankelevitz D, Li K, Xu D, Jirapatnakul A, Henschke C (2016) Lung cancer deaths in the National Lung Screening Trial attributed to cancers manifesting as part-solid nodules. AJR Am J Roentgenol. https://doi.org/10.2214/ajr.16.16930
Yip R, Wolf A, Tam K et al (2016) Outcomes of lung cancers manifesting as nonsolid nodules. Lung Cancer 97:35–42
NY-ELCAP Investigators (2007) CT screening for lung cancer: diagnoses resulting from the New York Early Lung Cancer Action Project. Radiology 243:239–249
International Early Lung Cancer Action Program I, Henschke CI, Yankelevitz DF et al (2006) Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 355:1763–1771
Travis WD, Brambilla E, Noguchi M et al (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 8:381–385
Travis W, Brambilla E, Burke A, Marx A, Nicholson A (2015) WHO classification of tumours of the lung, pleura, thymus and heart, 4th edn. WHO classification of tumours, vol 7. International Agency for Research on Cancer, Lyon
Vazquez M, Carter D, Brambilla E et al (2009) Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 64:148–154
Yankelevitz D, Reeves A, Kostis WJ, Zhao B, Henschke C (2000) Determination of malignancy in small pulmonary noules based on volumetrically determined growth rates: preliminary results. Radiology 217:251–256
Hasegawa M, Sone S, Takashima S et al (2000) Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 73:1252–1259
Detterbeck FC, Gibson CJ (2008) Turning gray: the natural history of lung cancer over time. J Thorac Oncol 3:781–792
Henschke C, Yankelevitz D, Yip R et al (2012) Lung cancers diagnosed at annual CT screening: volume doubling times. Radiology 263:578–583
Yanagawa M, Tanaka Y, Leung AN et al (2014) Prognostic importance of volumetric measurements in stage I lung adenocarcinoma. Radiology 272:557–567
Carter D, Vazquez M, Flieder DB et al (2007) Comparison of pathologic findings of baseline and annual repeat cancers diagnosed on CT screening. Lung Cancer 56:193–199
Ginsberg M, Grewal R, Heelan R (2007) Lung cancer. Radiol Clin N Am 45:21–43
Henschke C, McCauley D, Yankelevitz D et al (1999) Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 354:99–105
Henschke C, Yankelevitz D, Naidich D et al (2004) CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 231:164–168
Henschke C, Yip R, Yankelevitz DF, Smith JP (2013) Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 158:246–252
Moyer VA, US Preventive Services Task Force (2014) Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 160:330–338
American College of Radiology (2014) LungRADS. http://www.acrorg/Quality-Safety/Resources/LungRADS Accessed 28 Aug 2017
Yip R, Henschke C, Yankelevitz D, Boffetta P, Smith J, The International Early Lung Cancer Investigators (2015) The impact of the regimen of screening on lung cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev 24:201–208
Aberle D, Adams A, Berg C et al (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395–409
Horeweg N, van der Aalst CM, Thunnissen E et al (2013) Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 187:848–854
Field JK, Duffy SW, Baldwin DR et al (2016) UK lung cancer RCT pilot screening trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 71:161–170
Acknowledgements
The screenings in the I-ELCAP pooled database have been supported in part by National Institutes of Health R01-CA-63393l and R01-CA-78905; Department of Energy DE-FG02-96SF21260; The City of New York, Department of Health and Mental Hygiene; New York State Office of Science, Technology and Academic Research (NYSTAR); American Cancer Society; The Starr Foundation; The New York Community Trust; The Rogers Family Fund; The Foundation for Lung Cancer: Early Detection, Prevention, and Treatment (including an unrestricted gift in 2000–2003 from the Vector Group, the parent company of Liggett Tobacco); Dorothy R. Cohen Foundation, Jacob and Malka Goldfarb Charitable Foundation; Auen/Berger Foundation; Berger Foundation; Mills Peninsula Hospital Foundation, Tenet Healthcare Foundation; Ernest E. Stempel Foundation; Academic Medical Development Corporation; Columbia University Medical Center, Empire Blue Cross and Blue Shield; Eastman-Kodak Corporation; General Electric Corporation; Weill Medical College of Cornell University; Cornell University; New York Presbyterian Hospital; Swedish Hospital; Christiana Care Helen F. Graham Cancer Center; Holy Cross Hospital; Eisenhower Hospital; Jackson Memorial Hospital Health System; Evanston Northwestern Healthcare.
The I-ELCAP Investigators
Mount Sinai School of Medicine, New York, NY: Claudia I. Henschke, Principal Investigator, David F. Yankelevitz, Rowena Yip, Dongming Xu, Mary Salvatore, Raja Flores, Andrea Wolf; Weill Cornell Medical College: Dorothy I. McCauley, Mildred Chen, Daniel M. Libby, James P. Smith, Mark Pasmantier; Cornell University: Anthony P. Reeves; CBNS, City University of New York at Queens College, Queens, NY; Steven Markowitz, Albert Miller; Fundacion Instituto Valenciano de Oncologia, Valencia, Spain: Jose Cervera Deval; University of Toronto, Princess Margaret Hospital, Toronto, Canada: Heidi Roberts, Demetris Patsios; Azumi General Hospital, Nagano, Japan: Shusuke Sone, Takaomi Hanaoka; Clinica Universitaria de Navarra, Pamplona, Spain: Javier Zulueta, Juan P. de-Torres, Maria D. Lozano; Swedish Medical Center, Seattle, WA: Ralph Aye, Kristin Manning; Christiana Care, Helen F. Graham Cancer Center, Newark, DE: Thomas Bauer; National Cancer Institute Regina Elena, Rome, Italy: Stefano Canitano, Salvatore Giunta; St.Agnes Cancer Center, Baltimore, MD: Enser Cole; LungenZentrum Hirslanden, Zurich, Switzerland: Karl Klingler; Columbia University Medical Center, New York, NY: John H.M. Austin, Gregory D. N. Pearson; Hadassah Medical Organization, Jerusalem, Israel: Dorith Shaham; Holy Cross Hospital Cancer Institute, Silver Spring, MD: Cheryl Aylesworth; Nebraska Methodist Hospital, Omaha NE: Patrick Meyers; South Nassau Communities Hospital, Long Island, NY: Shahriyour Andaz; Eisenhower Lucy Curci Cancer Center, Rancho Mirage, CA; Davood Vafai; New York University Medical Center, New York, NY: David Naidich, Georgeann McGuinness; Dorothy E. Schneider Cancer Center, Mills-Peninsula Health Services, San Mateo, CA: Barry Sheppard; State University of New York at Stony Brook, Stony Brook, NY: Matthew Rifkin; ProHealth Care Regional Cancer Center, Waukesha & Oconomowoc Memorial Hospitals, Oconomowoc, WI: M. Kristin Thorsen, Richard Hansen; Maimonides Medical Center, Brooklyn, NY: Samuel Kopel; Wellstar Health System, Marietta GA: William Mayfield; St. Joseph Health Center, St. Charles, MO: Dan Luedke; Roswell Park Cancer Institute, Buffalo, NY: Donald Klippenstein, Alan Litwin, Peter A. Loud; Upstate Medical Center, Syracuse, NY: Leslie J. Kohman, Ernest M. Scalzetti; Jackson Memorial Hospital, University of Miami, Miami, FL; Richard Thurer, Nestor Villamizar; State University of New York, North Shore-Long Island Jewish Health System, New Hyde Park, NY: Arfa Khan, Rakesh Shah; The 5th Affiliated Hospital of Sun Yat-Sen University, Zhuhai, China: Xueguo Liu; Mercy Medical Center, Rockville Center, NY: Gary Herzog; Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan: Diana Yeh; National Cancer Institute of China, Beijing, China: Ning Wu; Staten Island University Hospital, Staten Island NY: Joseph Lowry, Mary Salvatore; Central Main Medical Center: Carmine Frumiento; Mount Sinai School of Medicine, New York, NY: David S. Mendelson; Georgia Institute for Lung Cancer Research, Atlanta, GA: Michael V. Smith; The Valley Hospital Cancer Center, Paramus NJ: Robert Korst; Health Group Physimed/McGill University, Montreal, CA: Jana Taylor; Memorial Sloan-Kettering Cancer Center, New York, NY: Michelle S. Ginsberg; John Muir Cancer Institute, Concord CA: Michaela Straznicka; Atlantic Health Morristown Memorial Hospital, Morristown NJ: Mark Widmann; Alta Bates Summit Medical Center, Berkeley CA: Gary Cecchi; New York Medical College, Valhalla, NY: Terence A.S. Matalon; St. Joseph’s Hospital, Atlanta GA: Paul Scheinberg; Mount Sinai Comprehensive Cancer Center, Miami Beach, FL: Shari-Lynn Odzer; Aurora St. Luke’s Medical Center, Milwaukee WI: David Olsen; City of Hope National Medical Center, Duarte, CA: Fred Grannis, Arnold Rotter; Evanston Northwestern Healthcare Medical Group, Evanston, IL: Daniel Ray; Greenwich Hospital, Greenwich, CT: David Mullen; Our Lady of Mercy Medical Center, Bronx, NY: Peter H. Wiernik; Baylor University Medical Center, Dallas TX: Edson H. Cheung; Sequoia Hospital, Redwood City CA: Melissa Lim; Glens Falls Hospital, Glens Falls NY: Louis DeCunzo; Atlantic Medical Imaging, Atlantic City NJ: Robert Glassberg; Karmanos Cancer Institute, Detroit, MI: Harvey Pass, Carmen Endress; Rush University, Chicago IL: Mark Yoder, Palmi Shah; Building Trades, Oak Ridge TN: Laura Welch; Sharp Memorial Hospital, San Diego, CA: Michael Kalafer; Newark Beth Israel Medical Center, Newark NJ Jeremy Green; Guthrie Cancer Center, Sayre PA: James Walsh, David Bertsch; Comprehensive Cancer Centers of the Desert, Palm Springs CA: Elmer Camacho; Dickstein Cancer Treatment Center, White Plains Hospital, White Plains NY: Cynthia Chin; Presbyterian Healthcare, Charlotte NC: James O’Brien; University of Toledo, Toledo OH: James C. Willey
Funding
This study is sponsored in part by the Flight Attendant Medical Research Institute (FAMRI) and a generous gift by Sonia Lasry Gardner given in loving memory of her father, Moise Lasry, to support lung cancer research, outreach and treatment. The FAMRI supports research on never smokers, including assessment of the benefit of screening never smokers.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Claudia Henschke.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two of the authors have significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported. This is the first paper to report on the estimated lead time provided by CT screening which has never been reported. It also addresses length-time bias which is found in all screening programs for any cancer. For both of these goals, all cancers resulting from I-ELCAP collaboration on CT screening for lung cancer from 1992 to 2014 are included. The topic of lead time and length bias has never been addressed for this cohort.
The article also provides an update on the long-term survival from 1992 to 2014. The Lancet 1999 article [29] reported on the first 27 lung cancers diagnosed between 1992 and 1999, but not long-term survival. The N Engl J Med 2006 article [18] (see erratum in N Engl J Med. 2008 Apr 24;358(17):1875. N Engl J Med. 2008 Apr 24;358(17):1862. N Engl J Med. 2008 Aug 21;359(8):877). The results of lung cancer manifesting as nonsolid and part-sold nodules from 2001 to 2014 have been reported in Radiology [13] and AJR [12].
Methodology
• prospective
• cohort
• multicentre study
Additional information
A correction to this article is available online at https://doi.org/10.1007/s00330-017-5234-9.
Rights and permissions
About this article
Cite this article
Henschke, C.I., Salvatore, M., Cham, M. et al. Baseline and annual repeat rounds of screening: implications for optimal regimens of screening. Eur Radiol 28, 1085–1094 (2018). https://doi.org/10.1007/s00330-017-5029-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5029-z