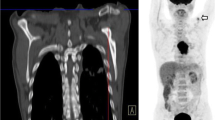
Abstract
Objectives
It is unknown whether restaging oesophageal cancer after neoadjuvant therapy with positron emission tomography-computed tomography (PET-CT) is more sensitive than contrast-enhanced CT for disease progression. We aimed to determine this and stratify risk.
Methods
This was a retrospective study of patients staged before neoadjuvant chemotherapy (NAC) by 18F-FDG PET-CT and restaged with CT or PET-CT in a single centre (2006-2014).
Results
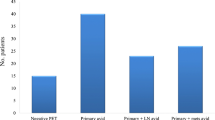
Three hundred and eighty-three patients were restaged (103 CT, 280 PET-CT). Incurable disease was detected by CT in 3 (2.91 %) and PET-CT in 17 (6.07 %). Despite restaging unsuspected incurable disease was encountered at surgery in 34/336 patients (10.1 %). PET-CT was more sensitive than CT (p = 0.005, McNemar’s test). A new classification of FDG-avid nodal stage (mN) before NAC (plus tumour FDG-avid length) predicted subsequent progression, independent of conventional nodal stage. The presence of FDG-avid nodes after NAC and an impassable tumour stratified risk of incurable disease at surgery into high (75.0 %; both risk factors), medium (22.4 %; either), and low risk (3.87 %; neither) groups (p < 0.001). Decision theory supported restaging PET-CT.
Conclusions
PET-CT is more sensitive than CT for detecting interval progression; however, it is insufficient in at least higher risk patients. mN stage and response (mNR) plus primary tumour characteristics can stratify this risk simply.
Key Points
• Restaging 18 F-FDG-PET-CT after neoadjuvant chemotherapy identifies metastases in 6 % of patients
• Restaging 18 F-FDG-PET-CT is more sensitive than CT for detecting interval progression
• Despite this, at surgery 10 % of patients had unsuspected incurable disease
• New concepts (FDG-avid nodal stage and response) plus tumour impassability stratify risk
• Higher risk (if not all) patients may benefit from additional restaging modalities



Similar content being viewed by others
References
Allum WH, Blazeby JM, Griffin SM et al (2011) Guidelines for the management of oesophageal and gastric cancer. Gut 60:1449–1472
Varghese TK Jr, Hofstetter WL, Rizk NP et al (2013) The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. Ann Thorac Surg 96:346–356
Stahl M, Budach W, Meyer HJ et al (2010) Esophageal cancer: clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21:v46–v49
National Comprehensive Cancer Guidelines (2014) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Esophageal and esophagogastric junction cancers. Version 1.2014, National Comprehensive Cancer Guidelines
Gillies RS, Middleton MR, Maynard ND et al (2011) Additional benefit of (1)(8)F-fluorodeoxyglucose integrated positron emission tomography/computed tomography in the staging of oesophageal cancer. Eur Radiol 21:274–280
Blencowe NS, Whistance RN, Strong S et al (2013) Evaluating the role of fluorodeoxyglucose positron emission tomography-computed tomography in multi-disciplinary team recommendations for oesophago-gastric cancer. Br J Cancer 109:1445–1450
Meyers BF, Downey RJ, Decker PA et al (2007) The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: results of the american college of surgeons oncology group Z0060 trial. J Thorac Cardiovasc Surg 133:738–745
Royal College of Surgeons Clinical Effectiveness Unit (2012) National Oesophago-Gastric Cancer Audit 2012. London, UK, Royal College Of Surgeons of England
Pennathur A, Gibson MK, Jobe BA et al (2013) Oesophageal carcinoma. Lancet 381:400–412
Urschel JD, Vasan H, Blewett CJ (2002) A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg 183:274–279
Campbell NP, Villaflor VM (2010) Neoadjuvant treatment of esophageal cancer. World J Gastroenterol 16:3793–3803
Blom RL, Schreurs WM, Belgers HJ et al (2011) The value of post-neoadjuvant therapy PET-CT in the detection of interval metastases in esophageal carcinoma. Eur J Surg Oncol 37:774–778
Bruzzi JF, Swisher SG, Truong MT et al (2007) Detection of interval distant metastases: clinical utility of integrated CT-PET imaging in patients with esophageal carcinoma after neoadjuvant therapy. Cancer 109:125–134
Gillies RS, Middleton MR, Han C et al (2012) Role of positron emission tomography-computed tomography in predicting survival after neoadjuvant chemotherapy and surgery for oesophageal adenocarcinoma. Br J Surg 99:239–245
Moon SH, Kim HS, Hyun SH et al (2014) Prediction of occult lymph node metastasis by metabolic parameters in patients with clinically N0 esophageal squamous cell carcinoma. J Nucl Med 55:743–748
Kwee RM (2010) Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18 F FDG PET: a systematic review. Radiology 254:707–717
Tamandl D, Gore RM, Fueger B, et al (2015) Change in volume parameters induced by neoadjuvant chemotherapy provide accurate prediction of overall survival after resection in patients with oesophageal cancer. Eur Radiol
Gillies RS, Middleton MR, Blesing C et al (2012) Metabolic response at repeat PET/CT predicts pathological response to neoadjuvant chemotherapy in oesophageal cancer. Eur Radiol 22:2035–2043
Findlay JM, Bradley, K.M., Maile, E.J., Braden, B., Maw, J., Phillips-Hughes, J., Maynard, N.D., Gillies, R.S., Middleton, M.R. (2015) Pragmatic staging of oesophageal cancer using decision theory involving selective endoscopic ultrasonography, PET and laparoscopy. British Journal of Surgery
Greene FL (2002) The american joint committee on cancer: updating the strategies in cancer staging. Bull Am Coll Surg 87:13–15
Rice TW, Blackstone EH, Rusch VW (2010) 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol 17:1721–1724
Edge SB, Compton CC (2010) The american joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Wahl RL, Jacene H, Kasamon Y et al (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50:122S–150S
Gillies RS, Simpkin A, Sgromo B et al (2011) Left thoracoabdominal esophagectomy: results from a single specialist center. Dis Esophagus 24:138–144
R Core Team (2013) R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing
Bland JM, Altman DG (1995) Multiple significance tests: the Bonferroni method. BMJ 310:170
Nelson LM, Bloch DA, Longstreth WT Jr et al (1998) Recursive partitioning for the identification of disease risk subgroups: a case-control study of subarachnoid hemorrhage. J Clin Epidemiol 51:199–209
Tu JV (1996) Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol 49:1225–1231
Pauker SG, Kassirer JP (1980) The threshold approach to clinical decision making. N Engl J Med 302:1109–1117
Rodgers M, Jobe BA, O'Rourke RW et al (2007) Case volume as a predictor of inpatient mortality after esophagectomy. Arch Surg 142:829–839
Statistics HE (2012) Main procedures and interventions: 4 character 2011-12, Hospital Episode Statistics Online
Park TG, Yu YD, Park BJ et al (2014) Implication of lymph node metastasis detected on 18 F-FDG PET/CT for surgical planning in patients with peripheral intrahepatic cholangiocarcinoma. Clin Nucl Med 39:1–7
Im HJ, Yoon HJ, Lee ES et al (2014) Prognostic implication of retrocrural lymph node involvement revealed by (18)F-FDG PET/CT in patients with uterine cervical cancer. Nucl Med Commun 35:268–275
Puli SR, Reddy JB, Bechtold ML et al (2008) Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 14:1479–1490
Lordick F, Ott K, Krause BJ et al (2007) PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 8:797–805
Doumou G, Siddique M, Tsoumpas C et al (2015) The precision of textural analysis in (18)F-FDG-PET scans of oesophageal cancer. Eur Radiol 25:2805–2812
De Cobelli F, Giganti F, Orsenigo E et al (2013) Apparent diffusion coefficient modifications in assessing gastro-oesophageal cancer response to neoadjuvant treatment: comparison with tumour regression grade at histology. Eur Radiol 23:2165–2174
van Rossum PS, van Hillegersberg R, Lever FM et al (2013) Imaging strategies in the management of oesophageal cancer: what's the role of MRI? Eur Radiol 23:1753–1765
Richardson JR, Khan OA (2012) In patients with radiologically-staged resectable oesophago-gastric junctional tumours, is diagnostic laparoscopy useful as an additional staging procedure? Int J Surg 10:198–202
Elliott JA, O'Farrell NJ, King S et al (2014) Value of CT-PET after neoadjuvant chemoradiation in the prediction of histological tumour regression, nodal status and survival in oesophageal adenocarcinoma. Br J Surg 101:1702–1711
Konieczny A, Meyer P, Schnider A et al (2013) Accuracy of multidetector-row CT for restaging after neoadjuvant treatment in patients with oesophageal cancer. Eur Radiol 23:2492–2502
Acknowledgements
The authors thank Rachael Styles for her assistance in data collection. JMF had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The scientific guarantor of this publication is Mr. John M Findlay. The authors of this manuscript declare relationships with the following companies: MRM has the following roles to disclose: Advisory/Consulting Role (payment to the individual) Amgen, BMS, GSK, Merck, Millennium and Roche. Research Funding (payment to the institution) from Amgen, AZ, BMS, Clovis, Eisai, GSK, Immunocore, Johnson & Johnson, Merck, Millennium, Novartis, Pfizer, Roche and Vertex. FVG is a paid consultant to Alliance Medical. The remaining authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, diagnostic or prognostic study performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Findlay, J.M., Gillies, R.S., Franklin, J.M. et al. Restaging oesophageal cancer after neoadjuvant therapy with 18F-FDG PET-CT: identifying interval metastases and predicting incurable disease at surgery. Eur Radiol 26, 3519–3533 (2016). https://doi.org/10.1007/s00330-016-4227-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4227-4