Abstract
Objectives
Due to the high prevalence of renal failure in transcatheter aortic valve replacement (TAVR) candidates, a non-contrast MR technique is desirable for pre-procedural planning. We sought to evaluate the feasibility of a novel, non-contrast, free-breathing, self-navigated three-dimensional (SN3D) MR sequence for imaging the aorta from its root to the iliofemoral run-off in comparison to non-contrast two-dimensional-balanced steady-state free-precession (2D-bSSFP) imaging.
Methods
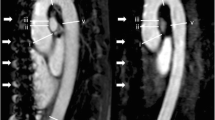
SN3D [field of view (FOV), 220-370 mm3; slice thickness, 1.15 mm; repetition/echo time (TR/TE), 3.1/1.5 ms; and flip angle, 115°] and 2D-bSSFP acquisitions (FOV, 340 mm; slice thickness, 6 mm; TR/TE, 2.3/1.1 ms; flip angle, 77°) were performed in 10 healthy subjects (all male; mean age, 30.3 ± 4.3 yrs) using a 1.5-T MRI system. Aortic root measurements and qualitative image ratings (four-point Likert-scale) were compared.
Results
The mean effective aortic annulus diameter was similar for 2D-bSSFP and SN3D (26.7 ± 0.7 vs. 26.1 ± 0.9 mm, p = 0.23). The mean image quality of 2D-bSSFP (4; IQR 3-4) was rated slightly higher (p = 0.03) than SN3D (3; IQR 2-4). The mean total acquisition time for SN3D imaging was 12.8 ± 2.4 min.
Conclusions
Our results suggest that a novel SN3D sequence allows rapid, free-breathing assessment of the aortic root and the aortoiliofemoral system without administration of contrast medium.
Key Points
• The prevalence of renal failure is high among TAVR candidates.
• Non-contrast 3D MR angiography allows for TAVR procedure planning.
• The self-navigated sequence provides a significantly reduced scanning time.



Similar content being viewed by others
Abbreviations
- 2D-bSSFP:
-
two-dimensional balanced steady-state free-precession
- 3D:
-
three-dimensional
- CNR:
-
contrast-to-noise ratio
- CT:
-
computed tomography
- Deff :
-
effective diameter
- FOV:
-
field of view
- MR:
-
magnetic resonance
- ROI:
-
region of interest
- SI:
-
signal intensity
- SN3D:
-
self-navigated 3-dimenasional
- TAVR:
-
transcatheter aortic valve replacement
References
Bloomfield GS, Gillam LD, Hahn RT et al (2012) A practical guide to multimodality imaging of transcatheter aortic valve replacement. J Am Coll Cardiol Img 5:441–455
Tsang W, Bateman MG, Weinert L et al (2012) Accuracy of aortic annular measurements obtained from three-dimensional echocardiography, CT and MRI: human in vitro and in vivo studies. Heart 98:1146–1152
Blanke P, Schoepf UJ, Leipsic JA (2013) CT in transcatheter aortic valve replacement. Radiology 269:650–669
Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA (2012) SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 6:366–380
Nguyen G, Leipsic J (2013) Cardiac computed tomography and computed tomography angiography in the evaluation of patients prior to transcatheter aortic valve implantation. Curr Opin Cardiology 28:497–504
Leipsic J, Wood D, Manders D et al (2009) The evolving role of MDCT in transcatheter aortic valve replacement: a radiologists' perspective. Am J Roentgenol 193:W214–W219
Lung B, Cachier A, Baron G et al (2005) Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 26:2714–2720
Vahanian A, Otto CM (2010) Risk stratification of patients with aortic stenosis. Eur Heart J 31:416–423
Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY et al (2009) SCCT guidelines for performance of coronary computed tomography angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 3:190–204
Jabbour A, Ismail TF, Moat N et al (2011) Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J Am Coll Cardiol 58:2165–2173
Barone-Rochette G, Pierard S, Seldrum S et al (2013) Aortic valve area, stroke volume, left ventricular hypertrophy, remodeling, and fibrosis in aortic stenosis assessed by cardiac magnetic resonance imaging: comparison between high and low gradient and normal and low flow aortic stenosis. Circ Cardiovasc Imaging 6:1009–1017
Debl K, Djavidani B, Seitz J et al (2005) Planimetry of aortic valve area in aortic stenosis by magnetic resonance imaging. Invest Radiol 40:631–636
Friedrich MG, Schulz-Menger J, Poetsch T, Pilz B, Uhlich F, Dietz R (2002) Quantification of valvular aortic stenosis by magnetic resonance imaging. Am Heart J 144:329–334
Agarwal R, Brunelli SM, Williams K, Mitchell MD, Feldman HI, Umscheid CA (2009) Gadolinium-based contrast agents and nephrogenic systemic fibrosis: a systematic review and meta-analysis. Nephrol Dial Transplant 24:856–863
Wang Y, Alkasab TK, Narin O et al (2011) Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines. Radiology 260:105–111
von Knobelsdorff-Brenkenhoff F, Gruettner H, Trauzeddel RF, Greiser A, Schulz-Menger J (2014) Comparison of native high-resolution 3D and contrast-enhanced MR angiography for assessing the thoracic aorta. Eur Heart J Cardiovasc Imaging 15:651–658
Piccini D, Monney P, Sierro C et al (2014) Respiratory Self-navigated Postcontrast Whole-Heart Coronary MR Angiography: Initial Experience in Patients. Radiology 270:378–386
Lai P, Bi X, Jerecic R, Li D (2009) A respiratory self-gating technique with 3D-translation compensation for free-breathing whole-heart coronary MRA. Magn Reson Med 62:731–738
Stehning C, Bornert P, Nehrke K, Eggers H, Stuber M (2005) Free-breathing whole-heart coronary MRA with 3D radial SSFP and self-navigated image reconstruction. Magn Reson Med 54:476–480
Piccini D, Littmann A, Nielles-Vallespin S, Zenge MO (2012) Respiratory self-navigation for whole-heart bright-blood coronary MRI: methods for robust isolation and automatic segmentation of the blood pool. Magn Reson Med 68:571–579
Expert Panel on MRS, Kanal E, Barkovich AJ et al (2013) ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging 37:501–530
Piccini D, Littmann A, Nielles-Vallespin S, Zenge MO (2011) Spiral phyllotaxis: the natural way to construct a 3D radial trajectory in MRI. Magn Reson Med 66:1049–1056
Pontone G, Andreini D, Bartorelli AL et al (2013) Comparison of accuracy of aortic root annulus assessment with cardiac magnetic resonance versus echocardiography and multidetector computed tomography in patients referred for transcatheter aortic valve implantation. Am J Cardiol 112:1790–1799
Blanke P, Russe M, Leipsic J et al (2012) Conformational pulsatile changes of the aortic annulus: impact on prosthesis sizing by computed tomography for transcatheter aortic valve replacement. J Am Coll Cardiol Intv 5:984–994
Thourani VH, Keeling WB, Sarin EL et al (2011) Impact of preoperative renal dysfunction on long-term survival for patients undergoing aortic valve replacement. Ann Thorac Surg 91:1798–1806
Nguyen TC, Babaliaros VC, Razavi SA et al (2013) Impact of varying degrees of renal dysfunction on transcatheter and surgical aortic valve replacement. J Thorac Cardiovasc Surg 146:1399–1406
Yamamoto M, Hayashida K, Mouillet G et al (2013) Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J Am Coll Cardiol 62:869–877
Barbanti M, Latib A, Sgroi C et al (2013) Acute kidney injury after transcatheter aortic valve implantation with self-expanding CoreValve prosthesis: results from a large multicentre Italian research project. Euro Interv 10:133–140
Burman ED, Keegan J, Kilner PJ (2008) Aortic root measurement by cardiovascular magnetic resonance: specification of planes and lines of measurement and corresponding normal values. Circ Cardiovasc Imaging 1:104–113
Koos R, Altiok E, Mahnken AH et al (2012) Evaluation of aortic root for definition of prosthesis size by magnetic resonance imaging and cardiac computed tomography: implications for transcatheter aortic valve implantation. Int J Cardiol 158:353–358
Acknowledgments
The scientific guarantor of this publication is UJS. The authors of this manuscript declare relationships with the following companies: UJS is a consultant for and/or receives research support from Bayer, Bracco, GE Healthcare, Medrad, and Siemens Healthcare. DHS is a consultant for St. Jude Medical. HM receives lecture fees from St. Jude Medical. DP and EM are employees of Siemens Healthcare Germany. WGR and MOZ are employees of Siemens Healthcare USA. The other authors declare that they have no competing interests. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects in this study. None of the study subjects or cohorts have been previously reported. Methodology: prospective, diagnostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renker, M., Varga-Szemes, A., Schoepf, U.J. et al. A non-contrast self-navigated 3-dimensional MR technique for aortic root and vascular access route assessment in the context of transcatheter aortic valve replacement: proof of concept. Eur Radiol 26, 951–958 (2016). https://doi.org/10.1007/s00330-015-3906-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3906-x