Abstract
Objective
The aim of this study was to establish and evaluate (colour Doppler-) high-resolution-ultrasound (hrUS) and bench-top magnetic resonance imaging (btMRI) as new methods to monitor experimental colitis.
Materials and methods
hrUS, btMRI and endoscopy were performed in mice without colitis (n = 15), in mice with acute colitis (n = 14) and in mice with acute colitis and simultaneous treatment with infliximab (n = 19).
Results
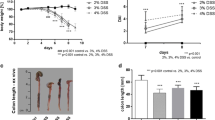
Determination of colon wall thickness using hrUS (32 MHz) and measurement of the cross-sectional colonic areas by btMRI allowed discrimination between the treatment groups (mean a vs. b vs. c – btMRI: 922 vs. 2051 vs. 1472 pixel, hrUS: 0.26 vs. 0.45 vs. 0.31 mm). btMRI, endoscopy, hrUS and colour Doppler-hrUS correlated to histological scoring (p < 0.05), while endoscopy and btMRI correlated to post-mortem colon length (p < 0.05).
Conclusions
The innovative in vivo techniques btMRI and hrUS are safe and technically feasible. They differentiate between distinct grades of colitis in an experimental setting, and correlate with established post-mortem parameters. In addition to endoscopic procedures, these techniques provide information regarding colon wall thickness and perfusion. Depending on the availability of these techniques, their application increases the value of in vivo monitoring in experimental acute colitis in small rodents.
Key points
• Improved in vivo monitoring might balance interindividual differences in murine colitis.
• In monitoring murine colitis, btMRI and hrUS are safe and technically feasible.
• Very short examination times underline the usefulness especially of hrUS.
• Results of btMRI and hrUS correlate with endoscopic and post-mortem findings.



Similar content being viewed by others
References
Loftus EV (2004) Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126:1504–1517
Baumgart DC, Sandborn WJ (2012) Crohn’s disease. Lancet 380:1590–1605
Brand S (2013) Moving the genetics of inflammatory bowel diseases from bench to bedside: first steps towards personalised medicine. Gut 62:1531–1533
Pohlmann A, Tilling LC, Robinson A et al (2009) Progression and variability of TNBS colitis-associated inflammation in rats assessed by contrast-enhanced and T2-weighted MRI. Inflamm Bowel Dis 15:534–545
Melgar S, Karlsson L, Rehnström E et al (2008) Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol 8:836–844
Wirtz S, Neufert C, Weigmann B et al (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2:541–546
Larsson AE, Melgar S, Rehnström E et al (2006) Magnetic resonance imaging of experimental mouse colitis and association with inflammatory activity. Inflamm Bowel Dis 12:478–485
Michael S, Keubler LM, Smoczek A et al (2013) Quantitative phenotyping of inflammatory bowel disease in the IL-10-deficient mouse by use of noninvasive magnetic resonance imaging. Inflamm Bowel Dis 19:185–193
Jelicks LA (2010) Imaging the gastrointestinal tract of small animals. J Neuroparasitol 1, N100504
Huang EH, Carter JJ, Whelan RL et al (2002) Colonoscopy in mice. Surg Endosc 16:22–24
Becker C, Fantini MC, Neurath MF (2006) High resolution colonoscopy in live mice. Nat Protoc 1:2900–2904
Scharl M, Leucht K, Frey-Wagner I et al (2011) Knock-out of β-glucosidase 2 has no influence on dextran sulfate sodium-induced colitis. Digestion 84:156–167
Lied GA, Milde AM, Nylund K et al (2012) Increased wall thickness using ultrasonography is associated with inflammation in an animal model of experimental colitis. Clin Exp Gastroenterol 5:195–201
Durkee BY, Weichert JP, Halberg RB (2010) Small animal micro-CT colonography. Methods 50:36–41
Melgar S, Gillberg P-G, Hockings PD et al (2007) High-throughput magnetic resonance imaging in murine colonic inflammation. Biochem Biophys Res Commun 355:1102–1107
Caysa H, Metz H, Mäder K et al (2011) Application of Benchtop-magnetic resonance imaging in a nude mouse tumor model. J Exp Clin Cancer Res 30:69
Besheer A, Caysa H, Metz H et al (2011) Benchtop-MRI for in vivo imaging using a macromolecular contrast agent based on hydroxyethyl starch (HES). Int J Pharm 417:196–203
Whittem CG, Williams AD, Williams CS (2010) Murine colitis modeling using dextran sulfate sodium (DSS). J Vis Exp 1652
Qiu W, Wu B, Wang X et al (2011) PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest 121:1722–1732
Shanmugam NKN, Ellenbogen S, Trebicka E et al (2012) Tumor necrosis factor α inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS One 7:e38136
Wirtz S, Becker C, Blumberg R et al (2002) Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J Immunol 168:411–420
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Neye H, Voderholzer W, Rickes S (2004) Evaluation of criteria for the activity of Crohn’s disease by power Doppler sonography. Digestive 67–72
Limberg B (1999) Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z Gastroenterol 37:495–508
Hommes DW, van Deventer SJ (2004) Endoscopy in inflammatory bowel diseases. Gastroenterology 126:1561–1573
Waldner MJ, Wirtz S, Neufert C et al (2011) Confocal laser endomicroscopy and narrow-band imaging-aided endoscopy for in vivo imaging of colitis and colon cancer in mice. Nat Protoc 6:1471–1481
Olson TJP, Halberg RB (2011) in Exp Small Anim Colonoscopy (Miskovitz, P.) (InTech)
Neurath MF, Wittkopf N, Wlodarski A et al (2010) Assessment of tumor development and wound healing using endoscopic techniques in mice. Gastroenterology 139:1837–1843.e1
Hoffmann JC, Preiss JC, Autschbach F et al (2008) Clinical practice guideline on diagnosis and treatment of Crohn’s disease. Z Gastroenterol 46:1094–1146
Bachmann C, Klibanov AL, Olson TS et al (2006) Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology 130:8–16
Wang H, Machtaler S, Bettinger T et al (2013) Molecular Imaging of Inflammation in Inflammatory Bowel Disease with a Clinically Translatable Dual-Selectin-targeted US Contrast Agent: Comparison with FDG PET/CT in a Mouse Model. Radiology 3:818–829
Stidham RW, Xu J, Johnson LA et al (2011) Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology 141:819–826.e1
Frericks BB, Wacker F, Loddenkemper C et al (2009) Magnetic resonance imaging of experimental inflammatory bowel disease: quantitative and qualitative analyses with histopathologic correlation in a rat model using the ultrasmall iron oxide SHU 555 C. Invest Radiol 44:23–30
Mustafi D, Fan X, Dougherty U et al (2010) High-resolution magnetic resonance colonography and dynamic contrast-enhanced magnetic resonance imaging in a murine model of colitis. Magn Reson Med 63:922–929
Charpentier C, Marion-Letellier R, Savoye G et al (2012) Magnetic resonance colonography in rats with TNBS-induced colitis: a feasibility and validation study. Inflamm Bowel Dis 18:1940–1949
Acknowledgments
Jens Walldorf and Martin Hermann contributed equally to this research.
The scientific guarantor of this publication is Jens Walldorf. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was not required because no studies on humans were performed. Approval from the institutional animal care committee was obtained. Methodology: experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Predefined positions of ultrasound examination of the colon (GIF 44 kb)
Rights and permissions
About this article
Cite this article
Walldorf, J., Hermann, M., Porzner, M. et al. In-vivo monitoring of acute DSS-Colitis using Colonoscopy, high resolution Ultrasound and bench-top Magnetic Resonance Imaging in Mice. Eur Radiol 25, 2984–2991 (2015). https://doi.org/10.1007/s00330-015-3714-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3714-3