Abstract
Objective
To analyse pelvic autonomous innervation with magnetic resonance imaging (MRI) in comparison with anatomical macroscopic dissection on cadavers.
Material and methods
Pelvic MRI was performed in eight adult human cadavers (five men and three women) using a total of four sequences each: T1, T1 fat saturation, T2, diffusion weighed. Images were analysed with segmentation software in order to extract nervous tissue. Key height points of the pelvis autonomous innervation were located in every specimen. Standardised pelvis dissections were then performed. Distances between the same key points and the three anatomical references forming a coordinate system were measured on MRIs and dissections. Concordance (Lin’s concordance correlation coefficient) between MRI and dissection was calculated.
Results
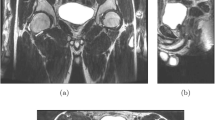
MRI acquisition allowed an adequate visualization of the autonomous innervation. Comparison between 3D MRI images and dissection showed concordant pictures. The statistical analysis showed a mean difference of less than 1 cm between MRI and dissection measures and a correct concordance correlation coefficient on at least two coordinates for each point.
Conclusion
Our acquisition and post-processing method demonstrated that MRI is suitable for detection of autonomous pelvic innervations and can offer a preoperative nerve cartography.
Key Points
• Nerve preservation is a hot topic in pelvic surgery
• High resolution MRI can show distal peripheral nerves
• Anatomo-radiological comparison shows good correlation between MRI and dissection
• 3D reconstructions of pelvic innervation were obtained with an original method
• This is a first step towards image-guided pelvic surgery




Similar content being viewed by others
References
Heald RJ, Moran BJ (1998) Embryology and anatomy of the rectum. Semin Surg Oncol 15(2):66–71
Porter GA, Soskolne CL, Yakimets WW, Newman SC (1998) Surgeon-related factors and outcome in rectal cancer. Ann Surg 227(2):157–167
Chalfin HJ, Dinizo M, Trock BJ et al (2012) Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int 110(11):1684–1689
Lange MM, Marijnen CA, Maas CP et al (2009) Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer 45(9):1578–1588
Marien T, Sankin A, Lepor H (2009) Factors predicting preservation of erectile function in men undergoing open radical retropubic prostatectomy. J Urol 181(4):1817–1822
Bertrand MM, Alsaid B, Droupy S, Benoit G, Prudhomme M (2013) Biomechanical origin of the Denonvilliers' fascia. Surg Radiol Anat 36(1):71–78
Alsaid B, Karam I, Bessede T et al (2010) Tridimensional computer-assisted anatomic dissection of posterolateral prostatic neurovascular bundles. Eur Urol 58(2):281–287
Heald RJ, Moran BJ, Brown G, Daniels IR (2004) Optimal total mesorectal excision for rectal cancer is by dissection in front of Denonvilliers' fascia. Br J Surg 91(1):121–123
Walsh PC, Epstein JI, Lowe FC (1987) Potency following radical prostatectomy with wide unilateral excision of the neurovascular bundle. J Urol 138(4):823–827
Lim KS, Tan CH (2012) Diffusion-weighted MRI of adult male pelvic cancers. Clin Radiol 67(9):899–908
Beets-Tan RG, Beets GL, Vliegen RF et al (2001) Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 357(9255):497–504
Brown G, Davies S, Williams GT et al (2004) Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer 91(1):23–29
Shihab OC, Heald RJ, Rullier E et al (2009) Defining the surgical planes on MRI improves surgery for cancer of the low rectum. Lancet Oncol 10(12):1207–1211
Mullerad M, Hricak H, Kuroiwa K et al (2005) Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J Urol 174(6):2158–2163
Filler AG, Howe FA, Hayes CE et al (1993) Magnetic resonance neurography. Lancet 341(8846):659–661
Takahara T, Hendrikse J, Yamashita T et al (2008) Diffusion-weighted MR neurography of the brachial plexus: feasibility study. Radiology 249(2):653–660
Panebianco V, Sciarra A, Osimani M et al (2009) 2D and 3D T2-weighted MR sequences for the assessment of neurovascular bundle changes after nerve-sparing radical retropubic prostatectomy with erectile function correlation. Eur Radiol 19(1):220–229
Laborde E (2012) Penile rehabilitation after radical prostatectomy: con. J Urol 187(1):16–17
Hedges JC (2012) Penile rehabilitation after radical prostatectomy: pro. J Urol 187(1):15–16
Prat-Pradal D, Metge L, Gagnard-Landra C, Mares P, Dauzat M, Godlewski G (2009) Anatomical basis of transgluteal pudendal nerve block. Surg Radiol Anat 31(4):289–293
Seewann A, Kooi EJ, Roosendaal SD, Barkhof F, van der Valk P, Geurts JJ (2009) Translating pathology in multiple sclerosis: the combination of postmortem imaging, histopathology and clinical findings. Acta Neurol Scand 119(6):349–355
Xu J, Zou Y, Zhang LH et al (2008) Postmortem MRI changes of the brains of the rats of different ages. Int J Neurosci 118(7):1039–1050
D'Arceuil H, de Crespigny A (2007) The effects of brain tissue decomposition on diffusion tensor imaging and tractography. Neuroimage 36(1):64–68
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128
Baader B, Herrmann M (2003) Topography of the pelvic autonomic nervous system and its potential impact on surgical intervention in the pelvis. Clin Anat 16(2):119–130
Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45(1):255–268
Mauroy B, Demondion X, Bizet B, Claret A, Mestdagh P, Hurt C (2007) The female inferior hypogastric (=pelvic) plexus: anatomical and radiological description of the plexus and its afferences–applications to pelvic surgery. Surg Radiol Anat 29(1):55–66
Lee SE, Hong SK, Han JH et al (2007) Significance of neurovascular bundle formation observed on preoperative magnetic resonance imaging regarding postoperative erectile function after nerve-sparing radical retropubic prostatectomy. Urology 69(3):510–514
Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M (1999) Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport 10(13):2817–2821
Finley DS, Ellingson BM, Natarajan S et al (2012) Diffusion tensor magnetic resonance tractography of the prostate: feasibility for mapping periprostatic fibers. Urology 80(1):219–223
Alsaid B, Bessede T, Diallo D et al (2011) Division of autonomic nerves within the neurovascular bundles distally into corpora cavernosa and corpus spongiosum components: immunohistochemical confirmation with three-dimensional reconstruction. Eur Urol 59(6):902–909
Chhabra A, Subhawong TK, Bizzell C, Flammang A, Soldatos T (2011) 3 T MR neurography using three-dimensional diffusion-weighted PSIF: technical issues and advantages. Skeletal Radiol 40(10):1355–1360
Chang KJ, Kamel IR, Macura KJ, Bluemke DA (2008) 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics 28(7):1983–1998
Hattori A, Suzuki N, Hashizume M et al (2003) A robotic surgery system (da Vinci) with image guided function–system architecture and cholecystectomy application. Stud Health Technol Inf 94:110–116
Alsaid B, Bessede T, Diallo D et al (2012) Computer-assisted anatomic dissection (CAAD): evolution, methodology and application in intra-pelvic innervation study. Surg Radiol Anat 34(8):721–729
Acknowledgements
The scientific guarantor of this publication is Prof. Jean Paul Beregi (Radiology Department, CHU de Nîmes, University Montpellier 1, Nimes, France). The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. M Demattei (Statistics Department CHU Nîmes) kindly provided statistical advice for this manuscript. Institutional review board approval was not required because this is an experimental study on cadavers. Methodology: experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertrand, M.M., Macri, F., Mazars, R. et al. MRI-based 3D pelvic autonomous innervation: a first step towards image-guided pelvic surgery. Eur Radiol 24, 1989–1997 (2014). https://doi.org/10.1007/s00330-014-3211-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3211-0