Abstract
Female Weddell seals (Leptonychotes weddellii) display a mixed capital-income breeding strategy, losing up to 40% of their body mass between birthing and weaning their pups. How and when they regain energy stores, however, remains to be fully explored. To better understand the foraging by lactating Weddell seals, we fitted time-depth recorders and head-mounted cameras on 26 seals in Erebus Bay, Ross Sea, for ~ 5 days in November and December 2018 and 2019. We aimed to (1) identify prey species and foraging depth and (2) investigate relationships between seal physiology and demographics and probability of foraging. We recorded 2782 dives, 903 of which were > 50 m, maximum depth was 449.3 m and maximum duration was 31.1 min. Pup age likely contributes to the probability of a lactating Weddell seal foraging (Est. = 1.21 (SD = 0.61), z = 1.97, p = 0.0484). Among 846 prey encounters, the most frequent prey items were crustaceans (46.2%) and Antarctic silverfish (Pleuragramma antarcticum, 19.0%); two encounters were observed with juvenile Antarctic toothfish (Dissostichus mawsoni, 0.2%). We identified substantial variability in foraging behaviour, individually and between locations, and found that lactating seals target many species and some may specialise on certain prey groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactation is one of the costliest activities in a mammal's life cycle (Sapriza 2019), and Weddell seals (Leptonychotes weddellii) have one of the longest lactation periods of any phocid (~ 50 d; Wheatley et al. 2006). Once thought to fast throughout the entire lactation period, Wheatley et al. (2008) found that Weddell seal mothers instead display a mixed capital-income breeding strategy, in which some females forage increasingly to offset the energetic costs associated with lactation.
Knowing the targeted prey of Weddell seals is critical to informing their role in an ecosystem, especially in the Ross Sea region where their contribution is ~ 42% of the world population for the species (LaRue et al. 2021). Information on prey can also aid in understanding how fat stores and condition are regained following an energetically expensive period, such as lactation (e.g., Beltran et al. 2017; Salas et al. 2017). Direct observation of seals feeding at the surface, scat and stomach content analysis, video recordings, and stable isotope analysis have revealed that Weddell seals consume a range of prey, including notothenioid fishes, cephalopods, and crustaceans (Testa et al. 1985; Burns et al. 1998; Fuiman et al. 2002; Ainley and Siniff 2009; Goetz et al. 2017; Rumolo et al. 2020). While otoliths of bony fishes, cephalopod beaks, and crustacean exoskeletons are detectable in scat or stomach analysis, soft tissue remains of many species can be overlooked (Burns et al. 1998). Furthermore, soft tissue species may be detected using stable isotope and DNA analyses. Still, the presence of these species in an animal’s diet can be confused as secondary ingestion, consumed by the prey rather than the predator (Burns et al. 1998; Goetz et al. 2017). One way to overcome these challenges in diet analyses is using animal-borne video recorders (ABVRs). ABVRs allow for the identification of prey species and observation of foraging tactics, which in combination with other animal-borne sensors, provide insight into diet and foraging depths or new foraging techniques (Davis et al. 1999; Fuiman et al. 2002; Foster-Dyer et al. 2023).
Most previous Weddell seal ABVR research has focused on males or non-breeding females (Davis et al. 1999, 2013; Fuiman et al. 2002; Madden et al. 2008), with few studies utilising ABVRs to understand the behaviours of lactating females. To fill this gap in our understanding, we used bio-logging methods to identify prey and characterize the foraging behaviours of free-ranging, lactating Weddell seals during early to mid-lactation over two years. Our aims were to identify and quantify prey species encountered, the dive depths at which they were encountered, and whether demographic (i.e., maternal age, pup age, or breeding history) or physiological factors (i.e., maternal mass or body condition) relate to a lactating Weddell seal foraging. Our study was motivated by the recent designation of the Ross Sea Region Marine Protected Area, which aims to protect “the [undefined] structure and function” of the ecosystem (Brooks et al. 2021). Given this, we wanted to understand if lactating Weddell seals could be seen predating upon Antarctic toothfish (Dissostichus mawsoni). We hypothesised that the main prey encountered would be Antarctic silverfish (Pleuragramma antarcticum), in alignment with previous Weddell seal diet studies in the Ross Sea (Burns et al. 1998; Goetz et al. 2017). We hypothesised that some seals may also predate upon Antarctic toothfish due to the energetic value they offer (Lenky et al. 2012), which may mitigate some of the high-energy demands of lactation (Ponganis and Stockard 2007; Pinkerton et al. 2008; Wheatley et al. 2008). We further hypothesised that females with older pups and those with lower body condition will be more likely to forage than females with younger pups and of higher body condition due to the energetic demands of lactation (Wheatley et al. 2008).
Materials and methods
Study area
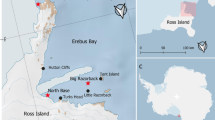
Our study took place in Erebus Bay (− 77.62°: − 77.87°S, 166.3°:167.0°E; Fig. 1), in the southern Ross Sea. Close to two research stations (McMurdo Station and Scott Base), Erebus Bay hosts the world's southernmost and well-studied population of breeding Weddell seals, which annually congregate among 8–14 breeding groups (or 'pupping colonies') along perennial tide cracks (Siniff et al. 1977; Rotella et al. 2009). This population has been studied since the late 1960s (Siniff et al. 1977) and has recently increased in size, with 760 pups born in the area in 2017 (Ainley et al. in press; Rotella unpubl. data). Erebus Bay is home to the largest aggregation of breeding Weddell seals found anywhere in the Antarctic; LaRue et al. (2021) estimated Erebus Bay to host ~ 35,000 adult and sub-adult female Weddell seals in 2011.
Map of Erebus Bay, in the southern Ross Sea, Antarctica (− 77.62° to − 77.87°S; 166.3° to 167.0°E). White dots indicate the six locations where lactating Weddell seals (Leptonychotes weddellii) were instrumented in November and December 2018 and 2019. Number of seals instrumented at each location were as follows: Pram Point (2019 n = 2), Hutton Cliffs (2018 n = 3, 2019 n = 1), North Base (2018 n = 4), Turks Head (2019 n = 3), Big Razorback (2018 n = 5, 2019 n = 2), Tent Island (2018 n = 6). Inset image shows a seal in 2018, fitted with a camera attached to its head, a magnetometer attached to upper back and an accelerometer attached under the jaw (Image: T. Iwata, 2018). Map made using Quantarctica (Matsuoka et al. 2021)
Animal deployments
We attached bio-logging devices to 26 lactating Weddell seals in November and December of 2018 (n = 18) and 2019 (n = 8) at six locations in Erebus Bay (Fig. 1). We selected seals based primarily on their apparent health (i.e., if they appeared alert and well) to prevent negative outcomes following sedation, and also based on their reproductive status. We further considered the age of their pup and whether their maternal age and breeding history were known (Rotella 2018). Since 1970, all seals born or encountered in the Erebus Bay population have been tagged with a livestock tag attached to each rear flipper (Siniff et al. 1977; Garrott et al. 2012).
The capture, immobilisation, and bio-logging device attachment procedures are described in Mellish et al. (2010) and Horning et al. (2019). Briefly, we captured each seal using a hoop net, then immobilised each with an initial dose of 2-mg kg−1 ketamine (100-mg mL−1) and 0.1-mg kg−1 midazolam (5-mg mL−1). As required, maintenance doses were administered at 0.5-mg kg−1 ketamine and 0.025-mg kg−1 midazolam intravenously in the extradural intervertebral venous sinus (Mellish et al. 2010). The animals were monitored by a qualified veterinarian throughout their sedation and recovery. While anaesthetised, we weighed each adult seal using a sling and tripod and measured girth and length using techniques described in Shero et al. (2014) and Beltran et al. (2018). We calculated each adult seal's 'fatness index,' used as a proxy for body condition, by dividing their axillary girth by their straight length (Stirling 1971; Sato et al. 2002). Data on location, demography and physiology gathered for each seal are reported in Online Resource Table SI1. We adhered bio-logging devices (cameras, TDRs, and accelerometers—see below) to the head and upper back fur using 5-min epoxy (Loctite Quickset Epoxy, 25 mL). Device details provided in Online Resource Table SI2.
We equipped each seal with a video camera (Little Leonardo DVL1300M130-VD3GT-2R (60 × 22 × 22 mm, 49 g, n = 24) or CATS-Cam Wireless CC v7–6.1.x (38 (diameter) × 130 mm, 280 g, n = 2) with a red light-emitting diode (LED) or infrared (IR) light (λmax = 850 nm) either on the top of the head (n = 23) or cheek (n = 3). Infrared light is likely invisible to Weddell seals and their prey due to their short-wavelength sensitive rod opsins, which are sensitive to blue-green light (λmax = 495–499 nm; Lythgoe and Dartnall 1970; Nealson 1981; Levenson et al. 2006). Seals equipped with Little Leonardo cameras were also fitted with a magnetometer time-depth recorder (TDR; Little Leonardo ORI1300-3MPD3GT (16.5 (diameter) × 90 mm, 42.5 g, n = 16) or W1000L-3MPD3GT (26 (diameter) × 176 mm, 120 g, n = 8)) on the upper back (inset in Fig. 1; Table SI2). CATS cameras include depth sensors, and thus no separate TDR was required. We also equipped the seals studied in 2018 with an accelerometer (Little Leonardo ORI2000-DG3T/ORI1300-DG3T (20 (diameter) × 73 mm, 52 g)) under the jaw that recorded depth and detected mouth-opening movement (mouth-opening and GPS data reported elsewhere: Iwata et al. in prep). Seven seals in 2019 were fitted on their back with a backward-facing camera (Little Leonardo DVL2000M130SW-4R (60 × 22 × 31 mm, 60 g), but little usable data were retrieved, which were not analysed further. Alongside the TDR, seals were also fitted with a VHF tag (ATS MM150 Marine Mammal Backmount, 13 (diameter) × 58 mm, 20 g) to allow us to relocate the animal and recover all devices.
The maximum total weight of all devices was less than 1% of each seal’s body mass, in line with acceptable field practice and technique (McMahon et al. 2008; Mazzaro and Dunn 2009; Horning et al. 2019). Devices were set to begin recording at least 12 h after deployment or after the seal reached an assigned depth (50–100 m). Doing this provided adequate time for the animals to recover from capture, resume normal behaviour and increase the likelihood of capturing movement data before the device memory or battery limits were reached. Our study required a short tagging period of ~ five days due to battery life and memory space limitations. After approximately five days (for details, see Online Resource Table SI1), we recaptured each seal and retrieved all equipment. Preliminary analysis of the 2018 season showed that seals with older pups (> 30 d) conducted deeper dives than those with younger pups. Because observing foraging behaviour was a key objective of our study, we modified our protocol and selected females with older pups in 2019.
All activities were approved by the New Zealand Department of Conservation, Ministry of Foreign Affairs and Trade, and NIWA's Animal Ethics Panel (Project number AEC210, END18301/Ross-RAMP: Ross Sea Research and Monitoring Program), and were carried out under permit number DOC-69331-MAR.
Video data collection
We analysed video footage using behavioural analysis software BORIS (Behavioural Observation Research Interactive Software; Friard and Gamba 2016). We defined the ethogram (an ethological catalogue that outlines behaviours exhibited by an animal; Friard and Gamba 2016) based on expected Weddell seal behaviour, quantifying 14 behaviours in total (see Online Resource Table SI3 for all behaviours quantified). We reviewed videos at normal speed and slowed to half-speed if detailed analysis was required, e.g., during prey encounters. A ‘prey encounter’ was defined as any time a seal attempted to consume an animal seen in the video; we selected this term as we are uncertain that the prey was consumed due to the camera angle and dark surrounding water. We also captured screenshots to assist with identification. Prey items were grouped by the lowest taxonomic class possible. We exported the summary output from BORIS into R version 4.0.4 (R Core Team 2021).
Dive analysis
We processed dive records (n = 25, one TDR had detached prior to recapture) in R version 4.0.4 (R Core Team 2021) using diveMove (version 1.5.3; Luque 2007). If duplicate dive records were present (i.e., when depth was recorded using both the back magnetometer and jaw accelerometer), we selected for analysis the instrument that provided the longest dataset. We corrected for surface inconsistencies and pressure drift in the tag data using the Igor Pro 8 Ethographer program (version 2.05; waterSurface extension, version 2.2). Dives were defined when the seal reached at least 10 m deep for at least 30 s (Beltran et al. 2021). For behaviours shallower than 10 m, the seal was considered at or near the surface, resting or interacting with its pup. Deep dives were defined as being > 50 m, whereas shallow dives were 10–50 m deep (Davis et al. 1999; Sato et al. 2002).
To understand foraging effort of individuals, we wanted to determine how many dives each seal performed, both overall and during a foraging bout. To determine the amount of time each seal was in the water, we excluded all data with a depth shallower than 1 m. We calculated each seal’s hourly dive rate by dividing the number of dives performed by hours in the water. We also calculated the number of foraging bouts each seal completed by visually identifying periods of recurring diving behaviour. A foraging bout was defined as any period of diving in which the seal reached at least 50 m depth at least once, as based on our prey encounter data and previous research (Sato et al. 2002), deep dives are more likely to represent foraging. Dives > 30 min from the previous dive were classified as a new bout. We calculated the average dive rate in a foraging bout by calculating the number of shallow (10–50 m) and deep dives (> 50 m) within each bout. To determine foraging effort, we calculated the average dive rate across all bouts performed by each seal and our study population.
Finally, we integrated the camera-derived prey encounters with the TDR-derived dive data to identify depth of prey encounters. Clock synchronisation was done manually by visually identifying dives and surface intervals in the video and TDR records that spanned the same length of time. Once matching dives were identified, we aligned the recording start times of each tag and imported the timestamps of each prey encounter into the TDR dataset. The depth range, mean, and standard deviation (SD) were then calculated for each prey type.
Statistical analysis
The probability of foraging while instrumented was determined by categorising the animals into two behavioural groups: ‘forager’ which were seals observed capturing prey in the video and/or diving deeper than 50 m on the TDR; and ‘non-forager’ which were seals that did not forage (i.e., did not reach 50 m depth or encounter prey while the camera or TDR were operational). This does not account for behaviours that may have occurred before or after instruments were deployed and we were unable to determine if foraging commenced prior to deployment. Two seals dived deeper than 10 m but did not reach the 50 m foraging dive threshold, and based on the frequency, depth, and durations of these dives, they were not considered to represent foraging (for details, see Online Resource Table SI4). No seals were excluded from the ‘forager’ category that performed long-duration shallow dives or exceptionally high numbers of shallow dives that may have indicated foraging at shallower depths. We further identified if seals were instrumented in early (< 20 d postpartum) or mid-lactation (≥ 20 d postpartum; Fig. 2), determined by the age of the female’s pup when she was instrumented. This was done to account for the physiological shift that occurs in the females at 20 d postpartum (Oftedal et al. 1987).
Deployment durations for each of the 26 lactating Weddell seals (Leptonychotes weddellii) equipped with seal-mounted cameras and time-depth recorders (TDRs) in Erebus Bay in 2018 and 2019. The red dashed line separates seal deployments during ‘early’ (< 20 d postpartum) and ‘mid-lactation’ (≥ 20 d postpartum) stages. The length of the bar indicates deployment duration, with the age of each seal’s pup beside each bar. Bars are coloured based on whether the seal was observed foraging in video or TDR analysis. The black dashed line separates the seals deployments in 2018 and 2019
We aimed to understand if demographic or physiological factors contributed to whether a lactating Weddell seal foraged while instrumented. We excluded seal deployments in 2019 from this analysis due to the sampling bias arising through our change in seal selection process (targeting females with older pups). Before testing the predictors of foraging status (‘forager’ or ‘non-forager’), we scaled and centred the predictors to ensure they were comparable and assessed relationships between our predictor variables (pup age, maternal mass, fatness, and number of previous pups) using R’s correlation function and corrplot (v. 0.92). We excluded the variable ‘number of previous pups’ due to the number of NAs. We further analysed multicollinearity within our predictors using a Variance Inflation Factor (VIF) analysis (car package; Fox and Weisberg 2019) and found all remaining covariates had a VIF score < 2.5.
We ran binomial logistic regressions in R using the lme4 package (Bates et al. 2015) to test foraging status (‘forager’ or ‘non-forager’) against pup age, maternal mass, and maternal fatness. We also assessed the inclusion of location as a random effect to address the potential for foraging access differing between locations. We ran a series of models with different combinations of the covariates and assessed model performance using AICc (MuMIn package; Bartoń 2024) to account for the small sample sizes. We assessed each model against a null using an Analysis of Variance (ANOVA). We calculated relative likelihood and Akaike weights for each model configuration using the qpcR package (v. 1.4–1) and identified the best-fit model by identifying the lowest AICc and highest evidenced ratio based on Akaike weights. We qualitatively describe some observed differences in dive behaviour between locations [see Online Resource Table SI4–SI7].
Results
Seal classification and statistical analysis
Of the 26 lactating seals, 16 were classified as a ‘forager’ and 10 as a ‘non-forager’ (Table 1). The only significant difference between the two groups was pup age (Paired t-test: t22.40 = 3.8209, p = 0.0009; Table 1). We also identified a significant difference in the average maternal mass of seals in early and mid-lactation (Paired t-test: t8.26 = 2.5096, p = 0.0355; Table 1). A binomial logistic regression (testing pup age, maternal mass, and body condition as predictors of a seal foraging) identified pup age as a likely contributor to foraging probability during lactation (Table 2, Online Resource Table SI8). The probability of foraging increased with pup age (Est. = 1.21 (SD = 0.61), z = 1.97, p = 0.0484; Fig. 3). The most parsimonious model, including only pup age and no random effect, provided the lowest AICc and highest Akaike weight and was the only model that was significantly different than the null (ANOVA: F16,17 = 2.70, p = 0.0264; Table 2).
Results from the binomial logistic regression model for a all seals and b only seals in 2018, identifying Weddell seal (Leptonychotes weddellii) pup age as an important factor contributing to whether its mother foraged while instrumented during lactation. Females were more likely to forage with increasing pup age. We report the results for only seals in 2018 due to the sampling bias produced by changing seal selection process between seasons but identified a significant trend across both groups
Diving behaviour
We recorded 2782 dives in total, consisting of 113 foraging bouts (Table 1, Online Resource Table SI4). Foraging seals completed 2775 dives, 903 deeper than 50 m (Table 1), and dived on average 173.4 times (SD = 179.2, n = 16) while instrumented. However, the number of dives varied greatly among individuals (Online Resource Table SI4). Maximum dive depth was 449.3 m and a maximum dive duration was 31.1 min (Online Resource Table SI4). On average, forager seals performed 7.8 deep dives (SD = 6.2, n = 16) and 6.6 shallow dives (SD = 4.0, n = 16) in a foraging bout (Online Resource Table SI4). Foraging effort varied individually—eight seals performed on average less than five deep dives in a foraging bout, while two seals performed on average more than 20 deep dives in a foraging bout (Online Resource Table SI4). Diving behaviour also varied by location—dives were deepest at Big Razorback and longest at Pram Point (Online Resource Tables SI5 and SI6). On average, foraging seals observed on video spent 27.7% (SD = 24.4, n = 11) of their time foraging benthically. However, this varied individually: one foraging seal did not visit the seafloor, whereas another spent 80% of their foraging time scanning the benthos (Online Resource Table SI7; for an example of behaviours displayed during benthic foraging see Foster-Dyer et al. 2023).
Prey encounters
We observed 846 prey encounters from nine foraging seals (Table 3), of which 84.1% were at depths > 50 m (Online Resource Table SI9). Prey were recorded from just below the ice down to 449.0 m, and the average depth of all prey encountered was 250.6 m (SD = 146.9, n = 846; Table 3, Fig. 4). The average depth of prey encounters varied by location: for example, at Turks Head 44.2% of the total prey encountered was above 50 m (Fig. 4). Comparatively, 0 and 0.3% of prey were encountered above 50 m at North Base and Big Razorback, respectively (Online Resource Table SI9). Average depth of prey encounters also varied individually (Online Resource Table SI10)—two individuals encountered prey at a mean depth of less than 100 m (48.6 m (SD = 19.8, n = 56) and 94.6 m (SD = 41.6, n = 33), respectively). The average depth of prey encountered by the remaining seven seals ranged between 152.6 m (SD = 97.4, n = 52) and 404.6 m (SD = 56.8, n = 56; Online Resource Table SI10).
Depth of prey encountered by lactating Weddell seals (Leptonychotes weddellii) in Erebus Bay, Antarctica. The average depth of encounters was 250.6 m highlighted by the dashed line. Violin bars are coloured by the location at which each seal was instrumented. Almost half (43.81%) of the prey encountered at Turks Head was shallower than 50 m
Prey types were identified in 576 encounters and included crustaceans (including Mysida, Decapoda, and Amphipoda), Antarctic silverfish, Antarctic toothfish, and bald notothen (Trematomus borchgrevinki; Table 3, Fig. 5). Crustaceans represented 46.2% of identified prey (including Antarctomysis maxima, Chorismus antarcticus, Notocrangon antarcticus, and Eusirus spp.) and, although encountered throughout the water column, were the deepest of any prey encountered (mean = 365.1 m, SD = 78.7, n = 391; Table 3). Most crustacean encounters were from two seals that encountered 257 and 130 crustaceans, respectively (Online Resource Table SI11), which were frequently encountered in quick succession (Fig. 6).
Still-images (taken from ABVR footage) of prey encountered by lactating Weddell seals (Leptonychotes weddellii) in Erebus Bay, Antarctica. Examples are: a five different Crustacea species, b Antarctic silverfish (Pleuragramma antarcticum) with the seal’s snout visible at the bottom of the image, c crocodile icefish (Channichthyidae), d two juvenile Antarctic toothfish (Dissostichus mawsoni), e bald notothen (Trematomus borchgrevinki), f unidentified eelpout (Zoarcidae), g the first octopus encounter (unidentified Octopod species), h the second octopus encounter (a different unidentified species). Image colour is due to the red LED light on the camera
Example of dive record and prey encounters from lactating Weddell seal (Leptonychotes weddellii) WS19-30. Black line represents the route travelled by the seal, circles represent prey encounter events, and colour of circle indicates prey type. a the complete TDR record, during which 311 prey encounters were observed at an average 404.6 m depth. The period that the camera was operational highlighted by red shaded area and red rectangle indicates dive period expanded below. b a closer view of the four dives when the camera was operational. WS19-30 encountered 257 crustaceans across the four dives. c the bottom of the first foraging dive where WS19-30 predated on 81 crustaceans while swimming near seafloor, encountering one prey approximately every 5.2 s. Inset: three examples of crustaceans encountered during the dive, identified as mysid shrimp (possibly Antarctomysis maxima)
We recorded 161 Antarctic silverfish (19.0% of prey) encountered by six of the nine successful foragers (Table 3). Most were encountered by one seal who consumed 132 silverfish (Online Resource Table SI11), which were often captured while the seal was ascending in the water column (Online Resource Figure SI12). We also observed one seal at Pram Point encountering two juvenile Antarctic toothfish (Fig. 5d) at a mean depth of 198.8 m (Table 3). We recorded encounters involving two Octopod species (which could not be identified further), and predation on smaller demersal fish species including Trematomus borchgrevinki, various unidentified Channichthyidae, and one unspecified Zoarcidae (Table 3, Fig. 5). We could not identify the species in a prey encounter 31.9% of the time (Table 3). We observed one seal encountering a T. borchgrevinki near the surface shortly after entering the water, and she appeared to ‘play’ with the prey—she captured the fish and chewed on it, before releasing the fish, recapturing, and releasing it once more (video available in Online Resource 2 SI13).
Discussion
We found individual variability in prey encounter and foraging behaviours consistent with lactating Weddell seals in Erebus Bay displaying mixed capital-income reproductive strategies, as previously reported (Wheatley et al. 2008; Beltran et al. 2017). We also found that some individuals appear to display prey specialisation, as reflected in scat analyses from this region and East Antarctica (Burns et al. 1998; Lake et al. 2003). Although lactating Weddell seals were once thought to forage “little, if at all” (pg. 95, Burns and Kooyman 2001), this study confirms that lactating Weddell seals do forage (Wheatley et al. 2008; Beltran et al. 2017) and that the likelihood of foraging increases from mid-lactation onwards.
Prey species and foraging behaviour
We present new evidence of direct predation on large numbers of crustaceans by lactating Weddell seals, which could influence the interpretation of past and future Weddell seal foraging studies. Crustaceans have been identified as prey for Weddell seals elsewhere in the Antarctic (Green and Burton 1987; Lake et al. 2003) but, to our knowledge, little evidence has indicated the same for seals in McMurdo Sound (Testa et al. 1985; Burns et al. 1998; Madden et al. 2008; Goetz et al. 2017). Previously, Burns et al. (1998) and Goetz et al. (2017) excluded any evidence of crustacean predation from their scat and stable isotope analysis (SIA), assuming that crustaceans were secondary prey (i.e., the prey of fish that were targeted by the seal) and that they were largely absent from the vicinity. Our results indicate direct crustacean predation, representing 46% of all prey encountered, and were targeted especially heavily by two seals, which captured 257 and 130 individuals each. This observation provides new insight into the prey encountered by Weddell seals in Erebus Bay, an area that hosts the largest concentration of breeding Weddell seals in Antarctica (LaRue et al. 2021). With an understanding that crustaceans identified through SIA may not merely represent secondary ingestion, future analysis of blood, fur, and whisker samples could be used to determine whether the seals that were observed predating upon large numbers of crustaceans do so outside of the lactation period when their foraging range is not limited.
In the present study, crustaceans were encountered most often, occurring in 46% of the prey encounters. Schaafsma et al. (2018) found crustaceans (Mysida, Decapoda, and Amphipoda) have the lowest energetic value of the prey encountered in our study (18.2–25.3 kJ g−1 dry weight (DW); see also Donnelly et al. 1994; Torres et al. 1994). The energy density of Antarctic silverfish varies between 21.76 and 27.93 kJ g−1 DW (Ainley et al. 2003; Van de Putte et al. 2010; Lenky et al. 2012; Ruck et al. 2014; summarised by Schaafsma et al. 2018), and Antarctic toothfish muscle contains the highest energy content of any known prey species of Weddell seals (29.94 kJ g−1 DW; Lenky et al. 2012; summarised by Schaafsma et al. 2018). It is possible that, given their mixed capital-income lactation strategy, lactating Weddell seals may not rely upon especially energy-dense prey species during this period as much of the early nutrients passed onto their young comes from mass gained prior to pupping (Wheatley et al. 2006, 2008). Alternatively, although we could not determine prey availability in Erebus Bay, lactating Weddell seals may require high-energy-dense prey but were observed encountering crustaceans most often in our study because of their high numbers and reduced availability of other prey species due to intraspecific competition (Hindell et al. 2002).
Behavioural plasticity or prey switching may be an important mechanism to support the energetic cost of milk production for some individuals during periods of high intraspecific competition. Fat content of Weddell seal milk begins at ~ 30% but increases to 60% through to mid-lactation (Kooyman 1981; Wheatley et al. 2008), perhaps necessitating the need to forage while lactating. The number of pups born in Erebus Bay increased in the years prior to our study (Ainley et al. in press; Rotella unpubl. data). As lactating Weddell seals are central-place foragers, predation pressure near breeding colonies can make preferred, high-energy density prey scarce within the foraging range if the predation is substantial relative to the prey density (Hindell et al. 2002). Testa et al. (1985); see also Ainley et al. (2021), showed that seals in McMurdo Sound (especially Erebus Bay) can reduce the prevalence of toothfish near breeding haul outs. Buckley (2013) also showed, in a natural experiment, that during a period when seal access to Erebus Bay was limited by multiyear ice (result of B-15A iceberg, 2001–2005; Siniff et al. 2008), the benthic fish fauna in the vicinity of our study area was quite different than it was during periods when seals are present in full numbers, providing further evidence that seal foraging can affect prey prevalence in some ecological circumstances. As seals in our study were observed encountering low-energy density crustaceans most often, we suggest this could be in response to changes in prey community structure near populous haul-out areas.
We recorded two encounters with Antarctic toothfish by one seal, which had the oldest pup of our study population (37 d) and was instrumented at Pram Point, close to the Ross and McMurdo Ice Shelves and farthest from the main Erebus Bay breeding area (see Fig. 1). The toothfish observed in our study were likely juveniles (approx. 50 cm in length; J. Eastman pers. comm.). Although toothfish are a known Weddell seal prey (Fuiman et al. 2002; Davis et al. 2004; Kim et al. 2011; Rumolo et al. 2020), the extent and timing of the Weddell seal ecological dependence on Antarctic toothfish remains unclear (Ponganis and Stockard 2007; Pinkerton et al. 2008; Ainley and Siniff 2009; Salas et al. 2017). This uncertainty was an important motivator for our study, given the recent designation of the Ross Sea Region Marine Protected Area and the uncertainty surrounding which demographic group predates on Antarctic toothfish (Ponganis and Stockard 2007; Pinkerton et al. 2008; Ainley and Siniff 2009; Salas et al. 2017). Ainley et al. (2013, 2021) suggested there may be a relationship between the scientific catch-per-unit-effort of toothfish and Weddell seal abundance in the southern McMurdo Sound area: namely, fewer toothfish were caught in areas with greater seal abundance. This decrease was linked to predation pressure or the possibility of toothfish moving away from areas of high seal occupation in response to noise produced by the highly vocal Weddell seals (Testa et al. 1985; Ainley et al. 2021). Further, Weddell seals and Antarctic toothfish have a complex intraguild relationship, in which seals both predate upon toothfish and compete with toothfish for many of the same prey (Fuiman et al. 2002; Fenaughty et al. 2003; Ainley et al. 2021). As both toothfish encounters in our study occurred at Pram Point where fewer Weddell seals pup, we suggest reduced seal occupation in the area may explain why toothfish were observed as prey in this location only. Fuiman et al. (2002) also found seals encountering toothfish only when well away from the main breeding aggregation. It is possible Antarctic toothfish were present at the other locations earlier in the season and were either driven away, outcompeted, or predated upon before our study commenced.
Drivers of foraging probability
Pup age likely contributes to whether a lactating Weddell seal foraged. This result was generally expected, given that pup age would inherently be correlated with the time elapsed since the mother was free to forage. Younger pups likely require more supervision than older, more independent pups. For example, Weddell seal pups enter the water from ~ 1 wk of age (Weitzner et al. 2021) but often require help from their mother to get out of the water (a behaviour observed in this study). This could impact pup survival if the adult is foraging when the pup enters the water. Furthermore, energetic requirements change as lactation progresses, including an increase in milk fat content (Kooyman 1981; Oftedal et al. 1987; Wheatley et al. 2008). Physiological demands fluctuate throughout the lactation period, as milk production (thus energy expenditure) changes during different stages of lactation. Wheatley et al. (2008) found fat and energy content of Weddell seal milk peaked around mid-lactation (20 d post-partum) and then decreased, while protein content increased throughout lactation. The authors suggest that females may begin foraging later in lactation to prevent loss of muscle stores as protein requirements of their pups increase (Wheatley et al. 2008). We postulate that these factors likely contribute to the relationship we observed between pup age and the probability of foraging.
We did not detect a relationship between foraging probability and body condition (measured using a fatness index) or maternal mass. Generally, in phocids, a larger body size allows a lactating female to fast longer due to the greater stored reserves and reduced metabolic overhead (Costa and Maresh 2022). Previous studies have linked maternal mass and/or fatness with diving behaviour of lactating females Weddell seals. Sato et al. (2002) found that thinner females spent a higher percentage of their day in deep foraging dives and suggested that supplementary foraging may compensate for inadequate mass gained prior to returning to the colony. Contrarily, Wheatley et al. (2008), who conducted their study in 2002–2003 when the seal population in Erebus Bay was low (owing to iceberg B-15A; Siniff et al. 2008), found that heavier females foraged more during lactation and suggested that, as heavier females can dive longer (Kooyman 1989), they may be able to exploit resources that lighter females cannot access. This may indicate an interesting compromise, in which larger/better condition females may be more able to take larger prey (like toothfish) while skinnier females may have greater need to do so. As such, we speculate there may be a peak in when seals predate on toothfish: small enough to be driven to but still strong enough to be capable of doing so. Importantly, indicators such as body mass or condition can vary greatly at an individual level, within and among breeding seasons, and can reflect environmental factors affecting the whole population (Schulz and Bowen 2004; Wheatley et al. 2006; Proffitt et al. 2007). The previously identified opposing findings may be associated with the inter-annual variability of foraging conditions outside of the lactation interval (e.g., Beltran et al. 2017). The lack of relationship observed in our study may be tied to our small sample size or short sampling duration (Bowen and Jonsen 2022).
Foraging behaviour varied between breeding locations. While the best-fit model did not include location, we report several differences in the foraging behaviours displayed at each location. We found that the deepest dives were performed at Big Razorback and that the longest duration dives (and often shallowest < 50 m) were performed at Pram Point (Online Resource Figure SI5 and Table SI6). Such spatial variation is likely linked to varying prey availability and access (Watanabe et al. 2003; Mitani et al. 2004). At Turks Head seals often captured prey at shallower depths than in other locations (Online Resource Table SI9). Furthermore, even though mid-lactation seals often foraged (Fig. 7), this was not true for Hutton Cliffs, where three of the four seals had pups older than 20 d, and yet none foraged during our study period. Interestingly, Hutton Cliffs hosts older, more experienced females (Hadley et al. 2008), suggesting that seals choosing to breed at such locations may be less reliant on foraging during lactation. Factors that may make it a desirable breeding location include predictable fast ice conditions and the shelter provided by the proximity to the Ross Island coastline.
The proportion of Weddell seals (Leptonychotes weddellii) instrumented at each location that were identified as a ‘forager’ through TDR and video analysis and those that were instrumented when their pups were at least 20 d old (in mid-lactation). The number (n = x) at the top of the bars refers to the number of seals instrumented at each location. Generally, proportions were equal across the locations, excluding Big Razorback (BR) and Hutton Cliffs (HC). At BR, more seals foraged than were in mid-lactation. At HC, although three seals were instrumented in mid-lactation, none were observed foraging. At Tent Island, one seal foraged before her pup was 20 d old and another did not forage despite having a pup that was 31-d of age
In conclusion, our study of Weddell seals in Erebus Bay during 2018–2019 found that some do forage while lactating and that foraging effort and probability varied individually. Pup age appeared to affect whether a lactating seal foraged, though there were outliers in our study population. Some individuals may specialize on certain prey groups and we found that crustaceans may feature more heavily in Weddell seal diet in Erebus Bay than previously thought. Though far less energy-dense than Antarctic toothfish or silverfish, targeting crustaceans could be an essential component of the successful breeding effort, allowing for individuals to consume large numbers of smaller prey efficiently, potentially offsetting some of the high energetic demands of lactation, and may also provide an important source of protein later in lactation. Future work should further explore the role of location and prey availability in determining foraging behaviour during the lactation window.
References
Ainley DG, Siniff DB (2009) The importance of Antarctic toothfish as prey of Weddell seals in the Ross sea. Antarct Sci 21:317–327. https://doi.org/10.1017/S0954102009001953
Ainley DG, Ballard G, Barton KJ, Karl BJ, Rau GH, Ribic CA, Wilson PR (2003) Spatial and temporal variation of diet within a presumed metapopulation of Adélie penguins. Condor 105:95–106. https://doi.org/10.1093/condor/105.1.95
Ainley DG, Nur N, Eastman JT, Ballard G, Parkinson CL, Evans CW, DeVries AL (2013) Decadal trends in abundance, size and condition of Antarctic toothfish in mcmurdo sound, Antarctica, 1972–2011. Fish Fish 14:343–363. https://doi.org/10.1111/j.1467-2979.2012.00474.x
Ainley DG, Cziko PA, Nur N, Rotella JJ, Eastman JT, LaRue M, Stirling I, Abrams PA (2021) Further evidence that Antarctic toothfish are important to Weddell seals. Antarct Sci 33:17–29. https://doi.org/10.1017/S0954102020000437
Ainley DG, Morandini V, Salas L, Nur N, Rotella J, Shanhun F, LaRue M, Foster-Dyer RTN, Parkinson C, Arrigo KR, van Dijken G, Beltran R, Kim S, Brooks C, Kooyman G, Ponganis P, Anderson D, Goetz KT (in press) Response of indicator species to changes in food web and ocean dynamics of the Ross sea, Antarctic science. Accepted for publication, Antarctica
Bartoń K (2024) MuMIn: multi-model inference. R package version 1.48.4, https://CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beltran RS, Testa JW, Burns JM (2017) An agent-based bioenergetics model for predicting impacts of environmental change on a top marine predator, the Weddell seal. Ecol Model 351:36–50
Beltran RS, Ruscher-Hill B, Kirkham AL, Burns JM (2018) An evaluation of three-dimensional photogrammetric and morphometric techniques for estimating volume and mass in Weddell seals Leptonychotes weddellii. PLoS ONE 13:e0189865. https://doi.org/10.1371/journal.pone.0189865
Beltran RS, Kilpatrick AM, Breed GA, Adachi T, Takahashi A, Naito Y, Robinson PW, Smith WO Jr, Kirkham AL, Burns JM (2021) Seasonal resource pulses and the foraging depth of a southern ocean top predator. Proc R Soc B 288:20202817. https://doi.org/10.1098/rspb.2020.2817
Bowen WD, Jonsen ID (2022) Foraging ecology and behavior. In: Costa DP, McHuron EA (eds) Ethology and behavioral ecology of phocids. Springer, Cham, pp 179–227. https://doi.org/10.1007/978-3-030-88923-4_6
Brooks CM, Bloom E, Kavanagh A, Nocito ES, Watters GM, Weller J (2021) The Ross sea, Antarctica: A highly protected MPA in international waters. Mar Policy 134:104795. https://doi.org/10.1016/j.marpol.2021.104795
Buckley BA (2013) Rapid change in shallow water fish species composition in an historically stable Antarctic environment. Antarct Sci 25:676–680. https://doi.org/10.1017/S0954102013000114
Burns JM, Kooyman GL (2001) Habitat use by Weddell seals and emperor penguins foraging in the Ross sea, Antarctica. Am Zool 41:90–98. https://doi.org/10.1093/icb/41.1.90
Burns JM, Trumble SJ, Castellini MA, Testa JW (1998) The diet of Weddell seals in mcmurdo sound, Antarctica as determined from scat collections and stable isotope analysis. Polar Biol 19:272–282. https://doi.org/10.1007/s003000050245
Costa DP, Maresh JL (2022) Reproductive energetics of phocids. In: Costa DP, McHuron EA (eds) Ethology and behavioral ecology of phocids. Springer, Cham, pp 281–309. https://doi.org/10.1007/978-3-030-88923-4_6
Davis RW, Fuiman LA, Williams TM, Collier SO, Hagey WP, Kanatous SB, Kohin S, Horning M (1999) Hunting behavior of a marine mammal beneath the Antarctic fast ice. Science 283:993–996. https://doi.org/10.1126/science.283.5404.99
Davis RW, Hagey W, Horning M (2004) Monitoring the behavior and multi-dimensional movements of Weddell seals using an animal-borne video and data recorder. Mem Natl Inst Polar Res Spec Issue 58:148–154
Davis RW, Fuiman LA, Madden KM, Williams TM (2013) Classification and behavior of free-ranging Weddell seal dives based on three-dimensional movements and video-recorded observations. Deep Sea Res Part II 88:65–77. https://doi.org/10.1016/j.dsr2.2012.07.006
Donnelly J, Torres JJ, Hopkins TL, Lancraft TM (1994) Chemical composition of Antarctic zooplankton during austral fall and winter. Polar Biol 14:171–183. https://doi.org/10.1007/BF00240522
Fenaughty JM, Stevens DW, Hanchet SM (2003) Diet of the Antarctic toothfish (Dissostichus mawsoni) from the Ross sea, Antarctica (Subarea 88.1). CCAMLR Sci 10:113–123
Foster-Dyer RTN, Goetz KT, Pinkerton M, Iwata T, Holser R, Michael SA, Pritchard C, Childerhouse S, Rotella J, Federwisch L, Costa D, LaRue MA (2023) First observations of Weddell seals foraging in sponges in Erebus Bay, Antarctica. Polar Biol 46:611–621. https://doi.org/10.1007/s00300-023-03149-1
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage Thousand, Oaks
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. https://doi.org/10.1111/2041-210X.12584
Fuiman L, Davis R, Williams T (2002) Behavior of midwater fishes under the Antarctic ice: observations by a predator. Mar Biol 140:815–822. https://doi.org/10.1007/s00227-001-0752-y
Garrott RA, Rotella JJ, Siniff DB, Parkinson CL, Stauffer GE (2012) Environmental variation and cohort effects in an Antarctic predator. Oikos 121:1027–1040. https://doi.org/10.1111/j.1600-0706.2011.19673.x
Goetz KT, Burns JM, Hückstӓdt LA, Shero MR, Costa DP (2017) Temporal variation in isotopic composition and diet of Weddell seals in the western Ross sea. Deep Sea Res Part II 140:36–44. https://doi.org/10.1016/j.dsr2.2016.05.017
Green K, Burton HR (1987) Seasonal and Geographical Variation in the Food of Weddell Seals, Leptonychotes-Weddelii, in Antarctica. Wildl Res 14(4):475–89. https://doi.org/10.1071/WR9870475
Hadley GL, Rotella JJ, Garrott RA (2008) Spatial variation in age-specific probabilities of first reproduction for Weddell seals. Oikos 117:1165–1174. https://doi.org/10.1111/j.0030-1299.2008.16623.x
Hindell MA, Harcourt R, Waas JR, Thompson D (2002) Fine-scale three-dimensional spatial use by diving, lactating female Weddell seals Leptonychotes weddellii. Mar Ecol Prog Ser 242:275–284. https://doi.org/10.3354/meps242275
Horning M, Andrews RD, Bishop AM, Boveng PL, Costa DP, Crocker DE, Haulena M, Hindell M, Hindle AG, Holser RR, Hooker SK, Hückstädt LA, Johnson S, Lea MA, McDonald BI, McMahon CR, Robinson PW, Sattler RL, Shuert CR, Steingass SM, Thompson D, Tuomi PA, Williams CL, Womble JN (2019) Best practice recommendations for the use of external telemetry devices on pinnipeds. Anim Biotelemetry 7:1–7. https://doi.org/10.1186/s40317-019-0182-6
Kim SZ, Ainley DG, Pennycook J, Eastman JT (2011) Antarctic toothfish heads found along tide cracks of the McMurdo ice shelf. Antarct Sci 23:469–470
Kooyman GL (1981) Weddell seal, consummate diver. Cambridge University Press, London
Kooyman GL (1989) Diverse divers: physiology and behaviour. Zoophysiology. Springer, Berlin
Lake SE, Burton HR, van den Hoff J (2003) Regional, temporal and fine-scale spatial variation in Weddell seal diet at four coastal locations in east Antarctica. Mar Ecol Prog Ser 254:293–305. https://doi.org/10.3354/meps254293
LaRue M, Salas L, Nur N, Ainley D, Stammerjohn S, Pennycook J, Dozier M, Saints J, Stamatiou K, Barrington L, Rotella J (2021) Insights from the first global population estimate of Weddell seals in Antarctica. Sci Adv 7:eabh3674. https://doi.org/10.1126/sciadv.abh3674
Lenky C, Eisert R, Oftedal OT, Metcalf V (2012) Proximate composition and energy density of nototheniid and myctophid fish in McMurdo sound and the Ross sea, Antarctica. Polar Biol 35:717–724. https://doi.org/10.1007/s00300-011-1116-9
Levenson DH, Ponganis PJ, Crognale MA, Deegan JF, Dizon A, Jacobs GH (2006) Visual pigments of marine carnivores: pinnipeds, polar bear, and sea otter. J Comp Physiol A 192:833–843. https://doi.org/10.1007/s00359-006-0121-x
Luque SP (2007) An Introduction to the diveMove package. R-News 7:8–14
Lynch M, Bodley K (2008) Phocids. In: West G, Heard D, Caulkett N (eds) Zoo animal and wildlife immobilization and anesthesia. Blackwell Publishing, Iowa, pp 459–468
Lythgoe JN, Dartnall HJA (1970) A “deep sea rhodopsin” in a mammal. Nature 227:955–956. https://doi.org/10.1038/227955a0
Madden KM, Fuiman LA, Williams TM, Davis RW (2008) Identification of foraging dives in free-ranging Weddell seals Leptonychotes weddellii: confirmation using video records. Mar Ecol Prog Ser 365:263–275. https://doi.org/10.3354/meps07396
Matsuoka K, Skoglund A, Roth G, de Pomereu J, Griffiths H, Headland R, Herried B, Katsumata K, Le Brocq A, Licht K, Morgan F (2021) Quantarctica, an integrated mapping environment for Antarctica, the southern ocean, and sub-Antarctic islands. Environ Model Softw 140:105015
Mazzaro LM, Dunn JL (2009) Descriptive account of long-term health and behavior of two satellite-tagged captive harbor seals Phoca vitulina. Endang Species Res 10:159–163. https://doi.org/10.3354/esr00190
McMahon CR, Field IC, Bradshaw CJ, White GC, Hindell MA (2008) Tracking and data–logging devices attached to elephant seals do not affect individual mass gain or survival. J Exp Mar Biol Ecol 360:71–77. https://doi.org/10.1016/j.jembe.2008.03.012
Mellish JAE, Tuomi PA, Hindle AG, Horning M (2010) Chemical immobilization of Weddell seals (Leptonychotes weddellii) by ketamine/midazolam combination. Veterinary Anaesth Analg 37:123–131. https://doi.org/10.1111/j.1467-2995.2009.00517.x
Mitani Y, Watanabe Y, Sato K, Cameron MF, Naito Y (2004) 3D diving behavior of Weddell seals with respect to prey accessibility and abundance. Mar Ecol Prog Ser 281:275–281. https://doi.org/10.3354/meps281275
Nealson KH (1981) Bioluminescence: current perspectives. CEPCO Division, Burgess Publishing Company, Minneapolis, p 165
Oftedal OT, Boness DJ, Tedman RA (1987) The behavior, physiology, and anatomy of lactation in the Pinnipedia. In: Genoways HH (ed) Current mammalogy. Springer, Boston, pp 175–245. https://doi.org/10.1007/978-1-4757-9909-5_6
Pinkerton MH, Dunn A, Hanchet SM (2008) Trophic overlap of Weddell seals (Leptonychotes weddellii) and Antarctic toothfish (Dissostichus mawsoni) in the Ross sea, Antarctica. Document WG-EMM-08/43, CCAMLR, Hobart, Australia.
Ponganis PJ, Stockard TK (2007) Short note: the Antarctic toothfish: how common a prey for Weddell seals? Antarct Sci 19:441. https://doi.org/10.1017/S0954102007000715
Proffitt KM, Garrott RA, Rotella JJ, Wheatley KE (2007) Environmental and senescent related variations in Weddell seal body mass: implications for age-specific reproductive performance. Oikos 116:1683–1690. https://doi.org/10.1111/j.0030-1299.2007.16139.x
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rotella JJ (2018) “Demographic data for Weddell seal colonies in Erebus Bay through the 2017 Antarctic field season.” U.S. Antarctic program (USAP) data center. https://doi.org/10.15784/601125
Rotella JJ, Link WA, Nichols JD, Hadley GL, Garrott RA, Proffitt KM (2009) An evaluation of density-dependent and density-independent influences on population growth rates in Weddell seals. Ecology 90:975–984. https://doi.org/10.1890/08-0971.1
Ruck KE, Steinberg DK, Canuel EA (2014) Regional differences in quality of krill and fish as prey along the Western Antarctic Peninsula. Mar Ecol Prog Ser 509:39–55. https://doi.org/10.3354/meps10868
Rumolo P, Zappes IA, Fabiani A, Barra M, Rakaj A, Palozzi R, Allegrucci G (2020) The diet of Weddell seals (Leptonychotes weddellii) in Terra Nova Bay using stable isotope analysis. Eur Zool J 87:94–104. https://doi.org/10.1080/24750263.2020.1720832
Salas L, Nur N, Ainley D, Burns J, Rotella J, Ballard G (2017) Coping with loss of large, energy-dense prey: a potential bottleneck for Weddell seals in the Ross sea. Ecol Appl 27:10–25
Sapriza FGR (2019) Lactation strategies and milk composition in pinnipeds. In: M’Handi N (ed) Lactation in farm animals: biology, physiological basis, nutritional requirements, and modelization. IntechOpen Veterinary Medicine and Science, London, pp 3–25. https://doi.org/10.5772/intechopen.85386
Sato K, Mitani Y, Cameron MF, Siniff DB, Watanabe Y, Naito Y (2002) Deep foraging dives in relation to the energy depletion of Weddell seal (Leptonychotes weddellii) mothers during lactation. Polar Biol 25:696–702. https://doi.org/10.1007/s00300-002-0406-7
Schaafsma FL, Cherel Y, Flores H, Van Franeker JA, Lea MA, Raymond B, Van De Putte AP (2018) The energetic value of zooplankton and nekton species of the southern ocean. Mar Biol 165:1–35. https://doi.org/10.1007/s00227-018-3386-z
Schulz TM, Bowen WD (2004) Pinniped lactation strategies: evaluation of data on maternal and offspring life history traits. Mar Mamm Sci 20:86–114. https://doi.org/10.1111/j.1748-7692.2004.tb01142.x
Shero MR, Pearson LE, Costa DP, Burns JM (2014) Improving the precision of our ecosystem callipers: a modified morphometric technique for estimating marine mammal mass and body composition. PLoS ONE 9:e91233. https://doi.org/10.1371/journal.pone.0091233
Siniff DB, DeMaster DP, Hofman RJ, Eberhardt LL (1977) An analysis of the dynamics of a Weddell seal population. Ecol Monogr 47:319–335. https://doi.org/10.2307/1942520
Siniff DB, Garrott RA, Rotella JJ, Fraser WR, Ainley DG (2008) Opinion: projecting the effects of environmental change on Antarctic seals. Antarct Sci 20:425–435. https://doi.org/10.1017/S0954102008001351
Stirling I (1971) Population dynamics of the Weddell seal (Leptonychotes weddelli) in McMurdo sound, Antarctica, 1966–1968. Antarctic Pinnipedia 18:141–161. https://doi.org/10.1029/AR018p0141
Testa JW, Siniff DB, Ross MJ, Winter JD (1985) Weddell seal—Antarctic cod interactions in McMurdo sound, Antarctica. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 561–565
Torres JJ, Donnelly J, Hopkins TL, Lancraft TM, Aarset AV, Ainley DG (1994) Proximate composition and overwintering strategies of Antarctic micronektonic Crustacea. Mar Ecol Prog Ser 113:221–232
Van de Putte AP, Jackson GD, Pakhomov E, Flores H, Volckaert FA (2010) Distribution of squid and fish in the pelagic zone of the Cosmonaut sea and Prydz Bay region during the BROKE-West campaign. Deep Sea Res Part II 57:956–967. https://doi.org/10.1016/j.dsr2.2008.02.015
Watanabe Y, Mitani Y, Sato K, Cameron MF, Naito Y (2003) Dive depths of Weddell seals in relation to vertical prey distribution as estimated by image data. Mar Ecol Prog Ser 252:283–288. https://doi.org/10.3354/meps252283
Weitzner EL, Pearson LE, Tomanek L, Liwanag HEM (2021) Early diving behavior in Weddell seal (Leptonychotes weddellii) pups. J Mammal 102:1000–1008. https://doi.org/10.1093/jmammal/gyab058
Wheatley KE, Bradshaw CJ, Davis LS, Harcourt RG, Hindell MA (2006) Influence of maternal mass and condition on energy transfer in Weddell seals. J Anim Ecol 75:724–733. https://doi.org/10.1111/j.1365-2656.2006.01093.x
Wheatley KE, Bradshaw CJ, Harcourt RG, Hindell MA (2008) Feast or famine: evidence for mixed capital–income breeding strategies in Weddell seals. Oecologia 155:11–20. https://doi.org/10.1007/s00442-007-0888-7
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Author contributions were as follows: KG and MP contributed to the study conception/design; KG, MP and DC contributed to funding acquisition; KG, TI, RH, CP, SC, SM and RFD contributed to fieldwork and/or data processing; RFD, KG, TI, RH, DA, and ML contributed to data analysis and interpretation; figures and the first draft of the manuscript were prepared by RFD; all authors commented on previous versions of the manuscript; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 9387 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Foster-Dyer, R.T.N., Goetz, K.T., Iwata, T. et al. Prey targeted by lactating Weddell seals (Leptonychotes weddellii) in Erebus Bay, Antarctica. Polar Biol (2024). https://doi.org/10.1007/s00300-024-03294-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00300-024-03294-1