Abstract
Spotted wolffish (Anarhichas minor) is a poorly understood species which is often captured as part of mixed demersal fisheries across its range. Abundance has declined in many regions and there is a need for greater knowledge on its biology. To improve our understanding of reproduction of A. minor, we investigated inter- and intra- annual differences in fecundity, the influence of condition on fecundity and time scale of ovary development. From 2006 to 2021, 150 females A. minor were sampled in Icelandic waters. Of these females, 73 were also used to estimate spawning time together with an additional 334 females from commercial catch and surveys from 2006 to 2023. Backwards extrapolation of oocyte size indicates that vitellogenesis begins in December and is likely completed after 8–10 months. There was no evidence of either intra- or inter-annual differences in fecundity, indicating that downregulation is minimal and that fecundity of A. minor is stable between years. A positive relationship between oocytes size and fish length was detected, while body condition and hepatosomatic index had only a small influence on fecundity in comparison with weight. There was a negative relationship between length and relative fecundity and the exponent of the fecundity–length relationship was lower than exponent of the weight–length relationship. Therefore, total egg production is likely not proportional to the spawning stock biomass of A. minor, and unusually, total egg production would decrease with increasing proportion of larger fish in the population. The spawning season was estimated to be from middle of August to middle of October with peak of spawning in September.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Teleost fish display a wide range of reproductive strategies, which encompass numerous traits such as, age/size at maturation, fecundity, egg size, spawning time, number of batches per season, level of parental care etc. Knowledge of these traits within a species, together with other aspects of their biology, can indicate the productivity and susceptibility of a particular species to changes in its environment, including effects of fishing pressure (Stobutzki et al. 2002; Hobday et al. 2011). Such knowledge can also help identifying vulnerable life stages to direct disturbance, such as selective effect of fisheries on spawning populations (Scott et al. 2006; Zemeckis et al. 2014; Gunnarsson et al. 2016), or indirect disturbance through specific effects of fishing gears, other anthropogenic activities, and climate change (de Mitcheson and Colin 2012; van Overzee and Rijnsdorp 2014). Within a population, reproductive traits are known to vary both spatially and temporally due to variation in environment including prey availability, temperature, fishing pressure and genotypes (Bagenal 1966; Kjesbu et al. 1998; Yoneda and Wright 2004; Óskarsson and Taggart 2006; Rideout and Morgan 2007; Stares et al. 2007; Tobin and Wright 2011; McElroy et al. 2013; dos Santos Schmidt et al. 2020). Therefore, it is important to monitor reproductive traits to understand the spatial and temporal variability of the reproductive biology of a particular species’ population within a particular environment and time frame.
There is an increasing awareness that spawning stock biomass (SSB) does not adequately represent the reproductive potential of a stock (Trippel 1999). Many fish exhibit hyperallometric mass scaling of fecundity (Barneche et al. 2018) which can lead to a non-linear relationship between SSB and total egg production (TEP) (Marshall et al. 1998). Therefore, in order to evaluate how a stock´s ability to produce viable eggs and larvae varies over time, an index encompassing a greater number of factors is needed (Trippel 1999). The TEP represents the maximum number of potential recruits to a population and is likely to better predict recruitment than SSB (Kraus et al. 2002; Marshall et al. 2003). To estimate TEP, knowledge of the concerned species fecundity is required. However, estimation of fecundity is often time consuming and time series are scarce. Fecundity can be estimated indirectly using relationships between fecundity and other variables such as length, weight, body condition and hepatosomatic index (Marshall et al. 2000; Lambert et al. 2003; Tomkiewicz et al. 2003), but of course, knowledge on how fecundity is affected by these variables is required.
Spotted wolffish (Anarhichas minor) is one of the four species of the genus Anarhichas. It is primarily found at depths of ~150–600 m and is distributed across the North Atlantic Ocean (Barsukov 1959). Females mature at length ranging from 55 to 85 cm and at 7–9 years old (Østvedt 1963; Beese and Kändler 1969; Templeman 1986; Gusev and Shevelev 1997; Gunnarsson et al. 2008). Female A. minor have an unusual ovary development strategy, in that, once oocytes reach the cortical alveolus (CA) stage, they will remain at this stage for several years prior to spawning for the first time (Gunnarsson et al. 2008). This oocyte development strategy has only been documented in two other species, Atlantic wolffish (Anarhichas lupus) and Greenland halibut (Reinhardtius hippoglossoides) (Gunnarsson et al. 2006; Kennedy et al. 2011).
Spotted wolffish (Anarhichas minor) is a determinate spawner with large demersal eggs (4–7 mm) that are spawned in a single batch with an incubation period of 800–1000 °C degree-days, depending on incubation temperature (Falk-Petersen et al. 1999). Spawning takes place during summer and autumn, depending on geographical area (Templeman 1986; Gunnarsson et al. 2008). Based upon the large egg size and the low temperatures in which they inhabit, it is likely that vitellogenesis is initiated several months before spawning. Just before spawning, three groups of oocytes are usually present in the ovary of A. minor, the vitellogenic oocytes which will be spawned at the next spawning opportunity, oocytes at the CA stage that will be spawned the following year and a stock of previtellogenic oocytes from which oocytes for future spawning events are recruited (Beese and Kändler 1969).
Fecundity of A. minor has previously been examined in Iceland, Canada, Greenland, Norway and and Russia (Maslov 1944; Beese and Kändler 1969; Templeman 1986; Gusev and Shevelev 1997; Gunnarsson et al. 2008). While spatial differences in fecundity were observed during these studies, A. minor of 60–120 cm have a fecundity of ~ 4 to 50 thousand eggs (Gunnarsson et al. 2008). However, none of the previous studies considered oocyte development stage which impacts the estimation of fecundity. As the ovary development proceeds, fish commonly decrease the number of vitellogenic oocytes through atresia (Vladykov 1956; Kurita et al. 2003; Skjæraasen et al. 2013). As a result, observed differences in fecundity between regions might be due to individual variation in oocytes development stage rather than spatial variation.
In the last decades, populations of A. minor have been declining. As a consequence, A. minor in the Northwest Atlantic has been listed as threatened by the Canadian Species at Risk Act (SARA) (Kulka et al. 2004). In European waters, A. minor has recently been classified as near threatened (Collette et al. 2015). In Icelandic waters, the species has been decreasing since 1996 and has now reached a historical low level. There is no targeted fishery for A. minor in Iceland, but they do have commercial value, and are mainly caught as bycatch in fisheries targeting other groundfish species. Spotted wolffish (A. minor) was added to the Icelandic Individual Transferable Quota (ITQ) system in 2018, however catches have largely exceeded advice since 2012 (MFRI 2023).
Spotted wolffish (A. minor) is a data poor species and there is an urgent need to gather information on its reproductive biology, population trends and harvest to implement proper conservation strategy to avoid its depletion. In the present study, we aim to improve the understanding of, A. minor reproductive biology and provide information which will be beneficial for conservation measures and management. Obtaining fecundity samples of A. minor can be difficult due to the low numbers for both the number of fish caught during surveys and the number of ungutted fish landed by the commercial fishery, sampling is therefore often opportunistic and difficult to plan. In addition, collecting fecundity samples too early in development can impact the results because of downregulation of fecundity. Therefore, it is necessary to have information on how the progress of vitellogenesis influences fecundity. The aim of the present study is to investigate (1) the temporal variation of fecundity over a period of 5 years (2006, 2008–2010 and 2019); (2) the effects of life-history traits such as condition, hepatosomatic index, length and weight on fecundity; (3) the temporal variation of fecundity within a year to assess how progress through vitellogenesis (upon measurement of oocyte size) impacts fecundity and (4) the distribution of spawning and timing of the spawning season of A. minor. This information will provide valuable biological inputs to the conservation strategy and management plan of this near threatened and data poor species.
Materials and methods
Sampling
A total of 150 female A. minor, at the vitellogenic stage of oocyte development, were collected. Samples were taken from 2006 to 2010 during the annual Icelandic autumn groundfish survey (AGFS) which takes place in October (n = 27) and from the commercial fishery (n = 89). Additional samples were taken during 2014 in the same survey, AGFS (n = 1) and the period of years, 2016 and 2018–2021, 2010 included from the Icelandic spring groundfish survey (IGFS) which takes place in February–March (n = 17) and from the commercial fishery (n = 16) (Table 1; Fig. 1). For each fish total length (L) (± 1 cm) was measured and for most of them, ungutted weight (W), gutted weight (Wg), liver and gonad weight were also measured to the nearest 1 g (Table 2). In 2006–2010, gonads were removed from each fish and placed in permeable plastic bags which were placed in 10% formalin, except for two gonads sampled during the IGFS which were analysed onboard. The ovaries were transported to the laboratory for estimation of fecundity and measurement of oocyte diameter. From 2014, no gonads were preserved in formalin, the ovaries sampled during the AGFS and IGFS were analysed onboard while the gonads sampled from the commercial catch in 2019 were analysed in the laboratory within 24 h after removal from the fish.
Estimation of fecundity
Fecundity was estimated gravimetrically (Bagenal and Braum 1970). All the oocytes were removed from the ovary, blended and washed in water. After the oocytes were drained, they were weighted (Wo), and two sub-samples weighted (Wosubs) counted manually with only oocytes > 2 mm being counted (nosubs). The aim was to have sub-samples with ~ 100 oocytes, which was estimated using the naked eye, thus usually contained more than 100 oocytes. Potential fecundity (F) was estimated by Eq. (1).
The mean of the two estimates of fecundity was used to determine F and used for further analysis. If the coefficient of variation between these two estimates was larger than 5%, a third subsample was taken. Relative fecundity (RF) was estimated using Eq. (2).
Fish body condition
Relative fish body condition (Kr) was calculated for each fish with Eq. (3), with the assumption that individuals with a greater weight at length have greater energy reserves. Gutted weight was used to rule out any effect of the weight of the stomach contents. Predicted gutted weight (Wpg) was based on the estimated length–weight relationship using data from the 148 females collected (Eq. 4). Hepatosomatic index (HSI) was estimated with Eq. (5) and gonadosomatic index (GSI) with Eq. (6).
Measurements of the oocytes
Oocyte diameters (OD) were measured for a total of 119 fish. Generally, A. minor oocytes are rather spherical in shape, however two measurements, major and minor axes of each oocyte were used to estimate the average oocyte diameter. Measurements were done using Leica image Q500 MC and Sigmascan except for those measured on-board during the IGFS where callipers were used. To estimate OD, the first ten vitellogenic oocytes from the first subsample from the fecundity estimation was used. In 2006, of the four gonads sampled, 30 oocytes (instead of ten) were measured for two fish and a total of 20 oocytes were measured for the other two. Since all ovaries collected during the AGFS and the commercial fishery from 2006 to 2010 were preserved in formalin, the oocyte size was corrected to obtain fresh oocyte diameter using the formula from Gunnarsson et al. (2022).
Spawning time
To estimate spawning time of the Ielandic population of A. minor, trends in GSI and OD was used. Additional, fish sampled in commercial catch of pre-spawning and spent females in August–November (268) and AGFS (139; Table 5) between 2006 and2023, were examined. Of these fish, 73 had also been used in the current fecundity study. To examine distribution of spawning, data from AGSF in the years 2006–2023 were location of females on aforementioned maturity stage was used as a proxy for spawning location and the bottom depth on station where they were caught was used to estimate the bottom depth of spawning.
Statistical analysis
To find the best model to describe fecundity log(F), stepwise linear regressions were performed using the following variables: log(L), log(Wg), HSI, Kr, year, month, period (see below) as an interaction with either log(Wg) or log(L), and day of the year, in the first model where year, month and period were treated as factors. It seemed that day of the year and length were not independent, therefore the data were split in two periods based on observed difference in relative fecundity between the months January–June (P1) and July–October (P2, Fig. 3). Oocyte diameter was excluded from the analysis as predictor variable for fecundity, as it was only measured for 119 fish, years with a sample size < 10 were also excluded in the first model (Table 1). As the variable years did not show a significant effect on fecundity, years with a sample < 10 were therefore included subsequent analysis. The most parsimonious model was selected based on Bayesian Information Criteria (BIC, Schwarz 1978; Sakamoto et al. 1986). The variables selected by the stepwise regression were used to estimate log(F) with linear regression. The same method was applied for estimation of RF, except that Wg was excluded and L was not transformed, in the stepwise linear regressions.
The influence on oocyte diameter was examined using the same approach. To examine the proportion of variance explained by each variable in the final model, an ANOVA was used. A t test was used to examine differences between exponents.
Weighted mean was used to estimate mean spawning depth.
All analysis and statistical tests were performed using R version 4.1.2 (R Project 2020).
Results
Potential fecundity
The observed potential fecundity (F) ranged from 2383 to 33,472 oocytes for 52 (Wg, 1049 g)–108 (Wg, 12,730 g) cm fish, respectively.
In the stepwise linear regressions, neither year, month, period, or day of the year, improved the model fit. Thus, the data collected during the different years were combined and the stepwise linear regressions were repeated using the same variables as before.
The best model to predict log(F) included log(Wg) and HSI, (Linear regression; F2,136 = 267.7, R2 = 0.80, p = < 0.0001; Table 3; Fig. 2a). Log(Wg) explained 79% of the variance in the model and HSI about 1%. To examine the influence of log(L) on log(F), Wg was excluded from the first model which then included log (L), Kr, HSI, day of the year, year (factor), season (factor) and month (factor). In this case the best model to predict log(F) included log(L), Kr and HSI, (Linear regression; F3,135 = 178.6, R2 = 0.80, p = < 0.0001; Table 3; Fig. 2b). Log(L) explained 76% of the variance in the model, Kr 3% and HSI 1%.
Correlation between a log Potential fecundity and log fish gutted weight (Wg), b log Potential fecundity and log fish total length (L). The crosses represent observed fecundity in the period January–Juni and the open circles in the period July–October. The black lines represent predicted values from the best linear regression where in b both mean of hepatosomatic index (HSI) and relative condition (Kr) was used with log (L), and the grey lines represent predicted fecundity when both variable’s mean (HSI and Kr) was decreased or increased of one SD (Tables 2, 3). The same approach applies for a, but the predicted variables were log (Wg) and HSI. The broken lines represent 95% confidence interval estimated when mean values of HSI and Kr were used
Relative fecundity
The relative fecundity (RF) ranged from 1.22 to 4.24 oocytes g−1 with a mean of 2.25 oocytes g−1. The best model to predict RF included L, HSI and Kr (Linear regression; F3,135 = 12.7, R2 = 0.22, p = < 0.0001; Table 3; Fig. 3). L explained 13% of the variance in the model, HSI 6% and Kr 4%. According to the model, if HSI and Kr were constant, the RF decrease 0.016 oocytes g−1 with increasing length cm−1.
Relationship between relative fecundity (RF, number of oocytes g−1) and l total length (L). The crosses represent observed fecundity in the period January–Juni and the open circles in the period July–October. The black lines represent predicted values from the best linear regression where both mean of hepatosomatic index (HSI) and relative condition (Kr) was used with L, and the grey lines represent predicted fecundity when both variable’s mean (HSI and Kr) was decreased or increased of one SD (Tables 2, 3). The dotted lines represent 95% confidence interval estimated when mean values of HSI and Kr were used. The dashed line represents predicted values based on data sampled in July–October (Linear Regression, RF = 3.793 + (− 0.023 × L) + (4.08 × 0.101))
There was a negative relationship between L and RF, which is in accordance with that the exponent of length in power function used to describe the F–L relationship (F = 0.1336 × L2.562; n = 150,R2 = 0.74) is significantly lower than the exponent in the Wg–L relationship which was 3.00 (Eq. 4; t test, t150 = 2.56; p < 0.0057; Fig. 4 and Table 4). Similarly, the exponent in power function used to describe the F–Wg relationship (F = 7.4412 × Wg0.8575, n = 148, R2 = 0.74; Fig. 5 and Table 4) was lower than 1, or 0.853 with 95% confidence interval (0.768–0.948), which means that heavier fish have lower RF than lighter ones (Le Bris et al. 2015).
Power regressions for potential fecundity (F) versus total length (L) and gutted weight (Wg) versus L.The crosses represent observed values in the period January–Juni and the open circles in the period July–October. The grey symbols are observed values for L–Wg relationship for spotted wolffish (Anarhichas minor) females, the line predicted values based on all data, the grey dotted line the period January–June and grey dashed line the period July–October. The same approach applies for the F–L relationship, but the symbols and the lines are black. See Table 4 for estimates for the lines
Potential fecundity versus gutted weight for fish captured in January-June (crosses) and July–October (open circles). Power regression lines (Table 4) for January–June (red line), July–October (blue line), and all data combined (black line) are shown together with 95% confidence intervals for all data combined (dotted line)
The interaction between season and length did not improve the final model to predict relative fecundity. However, according to Fig. 3. there is a difference in the RF–L relationship between periods or the data sampled in January–June (P1) and July–October (P2). Therefore, the data was spilt by seasons and each dataset analysed as the original dataset. For P1 data the stepwise regression only chooses HSI (p > 0.05) as predictor variable. Which was showed in the final model to be insignificant influence on RF–L relationship, explaining less than 1% of the variance in it. For P2 the best model included L and HSI (Linear regression; F2,71 = 10.99, R2 = 0.24, p = < 0.0001; RF = 3.7933 + (L × -0.023) + (HSI × 0.101); Fig. 3). The L explained 21% of the variance in the RF–L relationship and HSI 3%. According to the model, if HSI was constant, the RF decrease 0.023 oocytes g−1 with increasing length cm−1. The mean RF for P1 was 2.14 oocytes g−1 and for P2, 2.39 oocytes g−1 (Table 2).
There was no relationship between RF and L for the P1 data, correspondently there was no difference between the exponent in the power function between F–L and Wg–L relationships (t test, t 71 = 0.80; p > 0.05; Fig. 4; Table 4). However, when P2 data was used with same approach, RF–L relationship was negative and the exponent F–L and Wg–L relationships different (t test, t77 = 3.22; p < 0.0009; Fig. 4; Table 4).
Hepatosomatic and relative body condition indices
Hepatosomatic index was the highest in January (5.76%), from which it decreases steadily until May (3.68%). Afterwards, HSI values were relatively stable (Fig. 6a). Relative body condition generally increased from January (1.04) to June (1.10); It then decreased until October (0.89) when it reached its lower value (Fig. 6c).
To examine the influence of HSI on log(F)–log(Wg), mean of HSI was reduced or increased by one SD (Fig. 2a; Tables 2, 3). For F, the change resulted in an increase/decrease of 140–1040 oocytes or a 4.3% change depending on the weight of the fish. For a fish of average weight (5803 g), this resulted in 560 oocytes change. In the step-by-step regression model, good predictors for log(F)–log(L) and RF–L relationships, were beside (log)L or L, Kr and HSI (Table 3). Therefore, mean of both HSI and Kr was either increased or decreased by one SD to examine the influence of both HSI and Kr on log(F)–log(L) and RF–L relationships (Fig. 2b, 3; Tables 2, 3). For the log(F)–log(L) relationship the change was increase/decrease of 375–2605 oocytes depending on the length of the fish or a 11.4% change. For the average fish length (82.7 cm), this resulted in increase/decrease of 1200 oocytes. For the RF–L relationship the change was ± 0.017 oocytes g−1 or 0.6–0.9% depending on the L of the fish. For a fish of average L, the change was 0.7%.
Gonadosomatic index and Oocyte’s diameter
The mean gonadosomatic index generally increased from January (2.16%) onwards, until September when it reached its maximum (31.99%). It then decreased in October (16.16%). The largest increase between months was between August (10.63%) and September (Fig. 6b).
The mean oocyte diameter (OD) increased linearly from 2.61 mm in January to 5.80 mm in September. Oocyte diameter then decreased between September and October to 4.35 mm (Fig. 6d). To examine influence of month (factor), year (factor), day of year, HSI, Kr, and L on OD these variables were included in the first model. According to stepwise regression day of the year (ANOVA; F1108 = 103.76, p < 0.0001), L (ANOVA; F1108 = 6.14, p = 0.0148) and HSI (ANOVA; F1108 = 5.94, p = 0.0164) were the best predictors of OD in the final model. Day of the year explained 46% of the variance, L3% and HSI 3%. The final linear regression equation to predict OD was OD = 0.442 + (day of the year × 0.012) + (0.023 × L) + (− 0.186 × HIS), R2 = 0.51, F3108 = 38.1, p < 0.0001). When assuming constant HSI and L, the model predicts that oocyte diameter increases about 0.012 mm day−1 from the 25th of January to 9th of October. When using the model to predict OD, changing L and HSI by one SD. resulted in an increase/decrease by ~ 0.07 mm or 1.4–3.6% for the largest to the smallest oocytes, respectively (Table 2, Fig. 7). To examine the influence of length on oocyte size the day of the year was given the value 257 or middle of September and HSI its mean value in September or 3.5 and the equation above used with different length. The oocytes diameter in mm was 4.20, 4.43, 4.66, 4.89 and 5.12 for 60, 70, 80, 90 and 100 cm long fish, respectively. Accordingly, in middle of September, a 100 cm fish had, on average, oocytes ~ 22% larger than that of a 60 cm fish.
Evolution of the oocyte diameter by day of the year. The black line represents predicted values from linear regression where both mean of total length (L) and hepatosomatic index (HSI) were used with day of the year. The grey lines represent predicted values from liner regression where one standard deviation were either subtracted from HSI and L or added (Table 2). The dotted lines represent 95% confidence interval estimated when mean values of L and HSI were used
Time, depth and distribution of spawning
According to GSI and OD spawning begins in August with a peak in September and is ongoing in October (Fig. 6b, d). According to data from commercial catch, 18% of female.
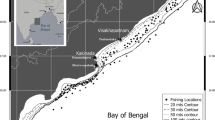
Anarhichas minor had spawned in the period 13th August to 22st August, in September 80% had spawn and all in October (Table 5). However, According to AGFS 72% was spent in October. However, in AGFS none pre-spawning female was catch after 17th October (Table 5). According to this the spawning period of A. minor in Iceland is from middle of August to middle October with peak of spawning in September. The bottom depth at spawning location in AGFS was at the range 133–610 m, mean depth was 345 m, SD = 138.7. A. minor spawn mainly in slope north-vest of Iceland, some spawning is also north of country and south-east of it, there seems to be none or little spawning south and south-west of Iceland (Fig. 8).
Location of capture of pre-spawning and spent female spotted wolffish (Anarhichas minor) from the annual autumn groundfish survey (AGFS) (filled circles) from 2006 to 2023. Crosses indicate stations where no pre-spawning or spent individuals were caught. Open circles show locations of spent individuals captured in the commercial fishery in October in the period 2008 to 2022
Discussion
Fecundity in fishes has been shown to be highly variable, both spatially and temporally (inter-annually) (Rideout and Morgan 2007) but there are also species which show limited variation between years (Kennedy and Ólafsson 2020). Despite the limited sampling, the current study indicates that fecundity of the A. minor did not vary between years. Various stages of oocyte development ranging from early to late vitellogenesis were observed, and no evidence for downregulation was found.
Comparison of results from the current study and previous studies indicates that fecundity of A. minor is similar across the North Atlantic (Fig. 9). The fecundity of A. minor in Barents Sea may be slightly higher than in Iceland. However, the fecundity data from Maslov (1944) consisted of 12 fish, of which, 3 were 120 cm long and therefore the results are not directly comparable to the present study. The data from Gusev and Shevelev (1997) are average fecundity of average length, therefore it is difficult to test statistically any differences between the Barents Sea and the present study. Similar, Beese and Kändler (1969) combined data from Barents Sea to west Greenland and in the study of Templeman (1986) only fecundity of three fishes was estimated (Fig. 9).
Fecundity of present study compared to other studies. The open circles (o) are data collected during the present study; (+ and ˟) represent data from the Barents Sea from Maslov (1944) and Gusev and Shevelev (1997), respectively; (♦) represent combined data from the Barents Sea to west Greenland (Beese and Kändler 1969) and (*) from Canada, (Templeman 1986). The line represents the power function correlation (F = 0.134 × L2.559; R2 = 0.74) of data in present study and the broken black lines represent the 95% confidential interval
Although period was not included in the final model to predict RF, presumably as it was overridden by Kr and HSI in the step regression, it was decided to divide the data into two separate periods as visual inspection of the data indicated a difference in RF between these two periods (Fig. 3). The trend in these two indices is different between periods, in P1 Kr is increasing while HSI is decreasing and in P2 Kr is decreasing while HSI seems to be stable. In P1 there was no relationship between RF and L, but in P2 this relationship was negative. Accordingly, there was no difference between the exponent in F–L and W–L relationship in P1, but it was in P2 (Fig. 4; Table 4). The reason for this difference is unclear but may be a result of the small sample size and further research is required.
Analysis of fish fecundity across 342 species showed that 79% displayed hyperallometric mass scaling of fecundity (Barneche et al. 2018) which equates to the exponent in the F–L relationship being greater than the exponent in the L–W relationship. The significance of a higher F–L exponent is that TEP of a population will increase when the average size of fish within the population increases (Pérez-Rodríguez et al. 2011; Dick et al. 2017). However, the current study showed that the exponent in the F–L relationship for A. minor was lower than the exponent in the L–W relationship (Fig. 4; Eq. 4; Table 4). Accordingly, for equal values of SSB, there is a negative relationship between TEP and the average length of A. minor within the stock. Negative relationship between RF and L has previously been documented for A. minor in the Barents Sea, for Atlantic wolffish around Iceland and mackerel icefish (Champsocephalus gunnari) in the South Atlantic Ocean (Gusev and Shevelev 1997; Militelli et al. 2015; Gunnarsson 2017).
As previously mentioned, 79% of the fish species included in the study by Barneche et al. (2018) displayed hyperallometric mass scaling of fecundity, however, when total reproductive energy was assessed by including data on energy content of eggs, 95% of species displayed hyperallometric mass scaling of reproductive energy output as the energy content of individual eggs increases with maternal size. Thus, even if TEP falls with increasing proportion of larger fish, the total reproductive energy output of the population would increase. The result of the current study suggests that this may be the case for A. minor as there was a significant correlation between oocyte size and fish size. However, this may be due to larger fish being at a later stage of development (Kjesbu 1994) rather than having larger eggs. It should be noted that in captive A. minor broodstock, no relationship has been found between female length and egg size (Falk-Petersen et al. 1999), however this has not been examined in wild individuals.
Weight was a better predictor of potential fecundity than length. This result is in accordance with findings from other studies of fish fecundity that denote that body weight is a better predictor of fecundity than length (Koops et al. 2004; Rideout and Morgan 2010; McElroy et al. 2016; Kennedy and Ólafsson 2020).
Hepatosomatic index was also included in the final model to predict fecundity, but only explained a small amount of variance, or about 1%, 3%, and 6% for F predicted with weight, F predicted with length and RF, respectively. Relative body condition index was included in the final model to predict F with length and RF, but again, the variance explained was small or 3% and 4%, respectively (Table 3). These results correspond with those of previous studies in which characteristics intended to measure energy reserves or “condition,”, added little in comparison to the factors reflecting body size (Óskarsson and Taggart 2006; Alonso-Fernández et al. 2009; Rideout and Morgan 2010; Gunnarsson 2017; Kennedy and Ólafsson 2020). The Kr was included in the final model to predict RF and the relationship was negative. This relationship might be because the fish is losing weight as they approach spawning and therefore RF is increasing while Kr is decreasing (Fig. 6c).
Hepatosomatic index was included in all final models to predict fecundity and a positive relationship between fecundity and HSI was detected in all instances (Table 3). The HSI decreased from January to May before stabilising, indicating that the weight loss in liver was proportionally lower than in carcass weight at least from July to October (Fig. 6a). Liver or body condition indices are presumed to reflect the energy reserves of the fish, but these different indices are not equivalent measure of bioenergetic condition (Pardoe et al. 2008). Protein and lipids, which can be both utilised for energy are stored differently in different species e.g. in Atlantic cod (Gadus morhua), most of the energy reserved for oocyte production, in the form of lipids, are stored in the liver while proteins are stored in the carcass (Black and Love 1986; Lambert and Dutil 1997). Whereas, in many flatfish species the liver is small, with little fat content and the main storage of lipids and protein is in the carcass (Tyler and Dunn 1976; Dawson and Grimm 1980). The mean hepatosomatic index of A. minor was about 4 which is between that of Atlantic cod and many flatfish species, such as winter flounder (Pseudopleuronectes americanus), which indicates that A. minor is storing proportionally more lipids in its liver that many flatfish species but less than Atlantic cod (Tyler and Dunn 1976; Pardoe et al. 2008). Despite A. minor using muscle as an energy store for lipids, the effect of Kr on fecundity was low. Part of the reason for this may be that the muscle water content may be variable between individuals which could mask the effect of condition i.e. some individuals with a high Kr, may simply have a high water content in their muscle (Lloret et al. 2014). In some species, the influence of body condition and hepatosomatic indices on fecundity peaks several months before spawning or at the beginning of vitellogenesis (Skjæraasen et al. 2006; Kennedy et al. 2007). This was not detected in the present study i.e., time did not influence the effect of condition on fecundity, perhaps due to low sample size during early vitellogenesis (Table 1).
In many species, fecundity decreases during ovary development due to a portion of the oocytes becoming atretic, this is known as downregulation, and refines the number of developing oocytes to bring fecundity in line with available energy reserves (Kurita et al. 2003). If ovary development is synchronized across the population, then it would be expected to see differences in fecundity at different points in time. However, none of the time variables, year, month, day of the year or period, were included in any final model to predict fecundity. This suggests that there was no temporal difference in fecundity indicating that levels of atresia were low. Although we did not assess atresia directly i.e., using histology, we assumed that atresia can be deduced indirectly using changes in fecundity over time. However, as sample sizes were low, it may be that such effects could not be detected. Many species typically exhibit an “atretic window” during development where atresia is at its highest intensity (Kjesbu et al. 1998; Óskarsson et al. 2002; Kennedy et al. 2007; Skjæraasen et al. 2013). Whether A. minor exhibit such a window is unknown, but in Atlantic wolffish the atresia primarily occurred during May–June when the mean oocyte diameter is about 2.1 mm and 2.7 mm, respectively (Gunnarsson 2017; Marine and Freshwater Research Institute (Iceland), unpublished data). If, A. minor have a window at a similar size, then this could explain the lack of downregulation as there were only a small number of fish at this stage of development. In addition, from June to October, synchronization on ovary development was low as indicated by the wide range of OD in these months (Fig. 6d). Atresia in, A. minor has been confirmed in captive fish but was only found in first-time spawners (Dupont Cyr et al. 2018). It certainly could be the case that, with, A. minor, there is little or no atresia. The magnitude of atresia within a species varies greatly and can depend on factors such as food availability, area and year, it also likely to vary across species due to life-history characteristics specific to each species (Tyler and Dunn 1976; Kjesbu et al. 1998; Óskarsson et al. 2002; Kennedy et al. 2008; Skjæraasen et al. 2013; McElroy et al. 2016).
Extrapolating oocyte sizes backwards suggests that vitellogenesis in, A. minor begins in December. Therefore, the duration of vitellogenesis would be in the range 8–10 months. This is a rather prolonged period for vitellogenesis compared to e.g., Atlantic wolffish or Atlantic cod where duration of vitellogenesis is about 4–6 months, but similar to lumpfish (Cyclopterus lumpus) where vitellogenesis is at least 8 months (Tveiten and Johnsen 1999; Yaragina 2010; Gunnarsson 2017; Kennedy and Ólafsson 2020).
Based upon the GSI and OD, data from commercial and AGFS catch, spawning appears to occur during August to October with peak of spawning in September. Spawning in August and September agrees with Gunnarsson et al. (2008) who documented spawning fish which were caught in the commercial fishery in August and September. However, of the mature fish landed from the fishery during 20th of September to November, all of them were spent despite pre-spawning fish being caught in AGFS during October, which is like the results in present study (Table 5). This mismatch between the fishery and the surveys suggest that maturity stages are not homogenously distributed through the population and given the mobility of the fishery, and the uncertainty around the migration timing and routes, it is difficult to be certain when the “main” or “peak” spawning occurs. Spawning season at Iceland begins later that in Russia, Norway and Canada, where spawning is mainly in July–August (Barsukov 1959; Østved 1963; Templeman 1986), but according to this study the main spawning in Iceland is from middle of August to middle of October.
In conclusion, there was a negative relationship between relative fecundity and length in July–October. There was no evidence to suggest intra- or inter-annual differences in fecundity, indicating little or no atresia during vitellogenesis in A. minor and thus estimates of fecundity collected at different points during vitellogenesis or different months of the year can be combined. Hepatosomatic index and condition had relatively small influence on fecundity. Spawning season is from middle of August to middle of October.
Data availability
The data used in the current study is available from the corresponding author on reasonable request.
References
Alonso-Fernández A, Vallejo AC, Saborido-Rey F et al (2009) Fecundity estimation of Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) of Georges Bank: application of the autodiametric method. Fish Res 99:47–54. https://doi.org/10.1016/j.fishres.2009.04.011
Bagenal TB (1966) The ecological and geographical aspects of the fecundity of the plaice. J Mar Biol Assoc United Kingdom 46:161–186
Bagenal TB, Braum E (1970) Egg and early life history. In Methods for assessment of fish production in fresh waters. In: Ricker WE (ed) IBP Handbook 3. Blakwell Science Publication, Oxford, pp 159–181
Barneche DR, Robertson DR, White CR, Marshall DJ (2018) Fish reproductive-energy output increases disproportionately with body size. Science 360:642–645. https://doi.org/10.1126/science.aao6868
Barsukov VV (1959) Wolffish family (Anarhichadidae). Indian National Documentation, New Delhi
Beese G, Kändler R (1969) Beitrage zur Biologie der drei nordatlantischen Katfischarten Anarhichas lupus L., A. minor Olafsen und A. denticulatus Krøyer. Berichte Der Dtsch Wissenschaftlichen Kommission Für Meeresforsch 20:21–59
Black D, Love RM (1986) The sequential mobilisation and restoration of energy reserves in tissues of Atlantic cod during starvation and refeeding. J Comp Physiol B 156:469–479. https://doi.org/10.1007/BF00691032
Collette B, Fernandes P, Heessen H (2015) Anarhichas minor (Europe assessment). The IUCN red list of threatened species 2015: e.T18263655A44739959. Accessed 08 Apr 2023
Dawson A, Grimm A (1980) Quantitative seasonal changes in the protein, lipid and energy content of the carcass, ovaries and liver of adult female plaice, Pleuronectes platessa L. J Fish Biol 16:493–504
de Mitcheson YS, Colin PL (eds) (2012) Reef fish spawning aggregations: biology, research and management. Springer, Netherlands
Dick EJ, Beyer S, Mangel M, Ralston S (2017) A meta-analysis of fecundity in rockfishes (genus Sebastes). Fish Res 187:73–85. https://doi.org/10.1016/j.fishres.2016.11.009
dos Santos Schmidt TC, Devine JA, Slotte A et al (2020) Environmental stressors may cause unpredicted, notably lagged life-history responses in adults of the planktivorous Atlantic herring. Prog Oceanogr 181:102257. https://doi.org/10.1016/j.pocean.2019.102257
Dupont Cyr BA, Tveiten H, Maltais D et al (2018) Photoperiod manipulation for the reproductive management of captive wolffish populations: Anarhichas minor and A. lupus. Aquac Int 26:1051–1065. https://doi.org/10.1007/s10499-018-0267-x
Falk-Petersen IB, Hansen TK, Fieler R, Sunde LM (1999) Cultivation of the spotted wolffish Anarhichas minor (Olafsen)—a new candidate for cold-water fish farming. Aquac Res 30:711–718
Gunnarsson Á (2017) Spatio-temporal variability in fecundity and atresia of Atlantic wolffish (Anarhichas lupus L.) population in Icelandic waters. Fish Res 195:214–221. https://doi.org/10.1016/j.fishres.2017.07.023
Gunnarsson Á, Hjörleifsson E, Thórarinsson K, Marteinsdóttir G (2006) Growth, maturity and fecundity of wolffish Anarhichas lupus L. in Icelandic waters. J Fish Biol 68:1158–1176. https://doi.org/10.1111/j.1095-8649.2006.00990
Gunnarsson Á, Hjörleifsson E, Thórarinsson K, Marteinsdóttir G (2008) Growth, maturity and fecundity of female spotted wolffish Anarhichas minor in Icelandic waters. J Fish Biol 73:1393–1406. https://doi.org/10.1111/j.1095-8649.2008.02017
Gunnarsson Á, Björnsson H, Elvarsson B, Pampoulie C (2016) Spatio-temporal variation in the reproduction timing of Atlantic Wolffish (Anarhichas lupus L) in Icelandic waters and its relationship with size. Fish Res 183:404–409. https://doi.org/10.1016/j.fishres.2016.07.002
Gunnarsson Á, Kennedy J, Magnússon Á et al (2022) Effect of formalin fixation on size and weight of Atlantic wolffish (Anarhichas lupus) and spotted wolffish (Anarhichas minor) oocytes. Fish Res 258:106515. https://doi.org/10.1016/j.fishres.2022.106515
Gusev EV, Shevelev MS (1997) New data on the individual fecundity of the wolffishes of the genus Anarhichas in the Barents Sea. J Ichthyol 37(5):381–388
Hobday AJ, Smith ADM, Stobutzki IC et al (2011) Ecological risk assessment for the effects of fishing. Fish Res 108:372–384. https://doi.org/10.1016/j.fishres.2011.01.013
Kennedy J, Ólafsson HG (2020) Intra-and interannual variation in fecundity and egg size of lumpfish (Cyclopterus lumpus) in Iceland. Fish Bull 118:250–267. https://doi.org/10.7755/FB.118.3.4
Kennedy J, Witthames PR, Nash RDM (2007) The concept of fecundity regulation in plaice (Pleuronectes platessa) tested on three Irish Sea spawning populations. Can J Fish Aquat Sci 64:587–601. https://doi.org/10.1139/f07-034
Kennedy J, Witthames PR, Nash RDM, Fox CJ (2008) Is fecundity in plaice (Pleuronectes platessa L.) down-regulated in response to reduced food intake during autumn? J Fish Biol 72:78–92. https://doi.org/10.1111/j.1095-8649.2007.01651.x
Kennedy J, Gundersen AC, Høines ÅS, Kjesbu OS (2011) Greenland halibut (Reinhardtius hippoglossoides) spawn annually but successive cohorts of oocytes develop over 2 years, complicating correct assessment of maturity. Can J Fish Aquat Sci 68:201–209. https://doi.org/10.1139/F10-149
Kjesbu OS (1994) Time of start of spawning in Atlantic cod (Gadus morhua) females in relation to vitellogenic oocyte diameter, temperature, fish length and condition. J Fish Biol 45:719–735
Kjesbu OS, Witthames PR, Solemdal P, Walker MG (1998) Temporal variations in the fecundity of Arcto-Norwegian cod (Gadus morhua) in response to natural changes in food and temperature. J Sea Res 40:303–321. https://doi.org/10.1016/s1385-1101(98)00029-x
Koops MA, Hutchings JA, McIntyre TM (2004) Testing hypotheses about fecundity, body size and maternal condition in fishes. Fish Fish 5:120–130. https://doi.org/10.1111/j.1467-2979.2004.00149.x
Kraus G, Tomkiewicz J, Koster FW (2002) Egg production of Baltic cod (Gadus morhua) in relation to variable sex ratio, maturity, and fecundity. Can J Fish Aquat Sci 59:1908–1920. https://doi.org/10.1139/f02-159
Kulka DW, Simpson MR, Hooper RG (2004) Changes in Distribution and Habitat Associations of Wolffish (Anarhichidae) in the Grand Banks and Labrador Shelf. Atl. Fish. Res. Doc. 04/113, p 44
Kurita Y, Meier S, Kjesbu OS (2003) Oocyte growth and fecundity regulation by atresia of Atlantic herring (Clupea harengus) in relation to body condition throughout the maturation cycle. J Sea Res 49:203–219. https://doi.org/10.1016/S1385-1101(03)00004-2
Lambert Y, Dutil J-D (1997) Can simple condition indices be used to monitor and quantify seasonal changes in the energy reserves of Atlantic cod (Gadus morhua)? Can J Fish Aquat Sci 54:104–112
Lambert Y, Yaragina NA, Kraus G et al (2003) Using environmental and biological indices as proxies for egg and larval production of marine fishes. J Northwest Atl Fish Sci 33:115–159
Le Bris A, Pershing AJ, Hernandez CM et al (2015) Modelling the effects of variation in reproductive traits on fish population resilience. Ices J Mar Sci 72:2590–2599. https://doi.org/10.1093/icesjms/fsv154
Lloret J, Shulman G, Love RM (2014) Condition and health indicators of exploited marine fishes. Wiley, New York
Marshall CT, Kjesbu OS, Yaragina NA et al (1998) Is spawner biomass a sensitive measure of the reproductive and recruitment potential of Northeast Arctic cod? Can J Fish Aquat Sci 55:1766–1783. https://doi.org/10.1139/cjfas-55-7-1766
Marshall CT, Yaragina NA, Ådlandsvik B, Dolgov AV (2000) Reconstructing the stock-recruit relationship for Northeast Arctic cod using a bioenergetic index of reproductive potential. Can J Fish Aquat Sci 57:2433–2442. https://doi.org/10.1139/cjfas-57-12-2433
Marshall CT, O’Brien L, Tomkiewicz J et al (2003) Developing alternative indices of reproductive potential for use in fisheries management: case studies for stocks spanning an information gradient. J Northwest Atl Fish Sci 33:161–190. https://doi.org/10.2960/J.v33.a8
Maslov NA (1944) The bottom-fishes of the Barents sea and their fisheries. Tr PINRO 8:3–186
McElroy WD, Wuenschel MJ, Press YK et al (2013) Differences in female individual reproductive potential among three stocks of winter flounder, Pseudopleuronectes americanus. J Sea Res 75:52–61. https://doi.org/10.1016/j.seares.2012.05.018
McElroy WD, Wuenschel MJ, Towle EK, McBride RS (2016) Spatial and annual variation in fecundity and oocyte atresia of yellowtail flounder, Limanda ferruginea, in U.S. waters. J Sea Res 107:76–89. https://doi.org/10.1016/j.seares.2015.06.015
MFRI (2023) Spotted wolffish Anarhichas minor. MFRI Assessment Reports 2023. https://www.hafogvatn.is/static/extras/images/16-spottedwolffish_tr1388171.pdf
Militelli MI, Macchi GJ, Rodrigues KA (2015) Maturity and fecundity of Champsocephalus gunnari, Chaenocephalus aceratus and Pseudochaenichthys georgianus in South Georgia and Shag Rocks Islands. Polar Sci 9:258–266. https://doi.org/10.1016/j.polar.2015.03.004
Óskarsson GJ, Taggart CT (2006) Fecundity variation in Icelandic summer-spawning herring and implications for reproductive potential. Ices J Mar Sci 63:493–503. https://doi.org/10.1016/j.icesjma.2005.10.002
Óskarsson GJ, Kjesbu OS, Slotte A (2002) Predictions of realised fecundity and spawning time in Norwegian spring-spawning herring (Clupea harengus). J Sea Res 48:59–79
Østvedt JO (1963) On the life history of the spotted catfish (Anarhichas minor Olafsen). Fisk Skr Ser Havundersøkelser 13:54–72
Pardoe H, Thórdarson G, Marteinsdóttir G (2008) Spatial and temporal trends in condition of Atlantic cod Gadus morhua on the Icelandic shelf. Mar Ecol Prog Ser 362:261–277. https://doi.org/10.3354/meps07409
Pérez-Rodríguez A, Morgan M, Rideout R et al (2011) Study of the relationship between total egg production, female spawning stock biomass, and recruitment of Flemish Cap cod (Gadus morhua). Ciencias Marine 37:675–687. https://doi.org/10.7773/cm.v37i4b.1785
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rideout RM, Morgan MJ (2007) Major changes in fecundity and the effect on population egg production for three species of north-west Atlantic flatfishes. J Fish Biol 70:1759–1779. https://doi.org/10.1111/j.1095-8649.2007.01448.x
Rideout RM, Morgan MJ (2010) Relationships between maternal body size, condition and potential fecundity of four north-west Atlantic demersal fishes. J Fish Biol 76:1379–1395. https://doi.org/10.1111/j.1095-8649.2010.02570.x
Sakamoto Y, Ishiguro M, Kitagawa G (1986) Akaike information criterion statistics. D. Reidel Publishing Company, Dordrecht
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464. https://doi.org/10.1214/aos/1176344136,MR0468014
Scott BE, Marteinsdottir G, Begg GA et al (2006) Effects of population size/age structure, condition and temporal dynamics of spawning on reproductive output in Atlantic cod (Gadus morhua). Ecol Modell 191:383–415. https://doi.org/10.1016/j.ecolmodel.2005.05.015
Skjæraasen JE, Nilsen T, Kjesbu OS (2006) Timing and determination of potential fecundity in Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 63:310–320. https://doi.org/10.1139/f05-218
Skjæraasen JE, Korsbrekke K, Kjesbu OS et al (2013) Size-, energy- and stage-dependent fecundity and the occurrence of atresia in the Northeast Arctic haddock Melanogrammus aeglefinus. Fish Res 138:120–127. https://doi.org/10.1016/j.fishres.2012.04.003
Stares JC, Rideout RM, Morgan MJ, Brattey J (2007) Did population collapse influence individual fecundity of northwest atlantic cod? Ices J Mar Sci 64:1338–1347. https://doi.org/10.1093/icesjms/fsm127
Stobutzki IC, Miller MJ, Heales DS, Brewer DT (2002) Sustainability of elasmobranchs caught as bycatchin a tropical prawn (shrimp) trawl fishery. Fish Bull 100:800–821
Templeman W (1986) Contribution to biology of the spotted wolffish (Anarhichas minor) in the Northwest Atlantic. Northwest Atl Fish Organ Sci Counc Stud 7:47–55
Tobin D, Wright PJ (2011) Temperature effects on female maturation in a temperate marine fish. J Exp Mar Bio Ecol 403:9–13. https://doi.org/10.1016/j.jembe.2011.03.018
Tomkiewicz J, Morgan MJ, Burnett J, Saborido-Ray F (2003) Available information for estimating reproductive potential of Northwest Atlantic groundfish stocks. J Northwest Atl Fish Sci 33:1–21
Trippel EA (1999) Estimation of stock reproductive potential: history and challenges for Canadian Atlantic gadoid stock assessments. J Northwest Atl Fish Sci 25:61–81
Tveiten H, Johnsen HK (1999) Temperature experienced during vitellogenesis influences ovarian maturation and the timing of ovulation in common wolffish. J Fish Biol 55:809–819
Tyler AV, Dunn RS (1976) Ration, growth, and measures of somatic and organ condition in relation to meal frequency in winter flounder, Pseudopleuronectes americanus, with hypotheses regarding population homeostasis. J Fish Res Board Canada 33:63–75. https://doi.org/10.1139/f76-008
van Overzee HMJ, Rijnsdorp AD (2014) Effects of fishing during the spawning period: implications for sustainable management. Rev Fish Biol Fish 25:65–83. https://doi.org/10.1007/s11160-014-9370-x
Vladykov VD (1956) Fecundity of wild speckled trout (Salaelinus fontinalis) in Quebec Lakes. J Fish Res Board Can 13:799–841
Yaragina NA (2010) Biological parameters of immature, ripening, and non-reproductive, mature northeast Arctic cod in 1984–2006. ICES J Mar Sci 67:2033–2041. https://doi.org/10.1093/icesjms/fsq059
Yoneda M, Wright PJ (2004) Temporal and spatial variation in reproductive investment of Atlantic cod Gadus morhua in the northern North Sea and Scottish west coast. Mar Ecol Prog Ser 276:237–248. https://doi.org/10.3354/meps276237
Zemeckis DR, Dean MJ, Cadrin SX (2014) Spawning dynamics and associated management implications for Atlantic Cod. N Am J Fish Manag 34:424–442. https://doi.org/10.1080/02755947.2014.882456
Acknowledgements
We thank Christophe Pampoulie and four anonymous reviewers for valuable comments on the manuscript.
Author information
Authors and Affiliations
Contributions
ÁG designed the study, carried out the laboratory work, analysed the data and wrote the first draft. JK participated in the analysis of data and writing of the manuscript, BE performed the statistical analysis of the data and participated in the writing. ARG data handing and reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The sampling was carried out in strict accordance with the relevant Icelandic legislation on animal welfare for Welfare of Experimental Animals. The authors therefore declare that all applicable international, national and/or institutional guidelines for sampling, care, and experimental use of organisms for the study have been followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gunnarsson, Á., Kennedy, J., Elvarsson, B. et al. Investigating temporal variability and influence of condition on fecundity and spawning of spotted wolffish (Anarhichas minor) in Icelandic waters. Polar Biol 47, 263–277 (2024). https://doi.org/10.1007/s00300-024-03229-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-024-03229-w