Abstract
Antarctic marine ecosystems are largely thought to be among the planet’s least impacted, yet habitats adjacent to research stations can be heavily polluted. Despite long-term monitoring and remediation being high priorities for international environmental protection, the ecological responses to contaminants and stressors remains poorly characterised, limiting our ability to manage and reduce human impacts. This study compares epifaunal community composition at two sites close to Scott Base with a reference site further away. We couple these data with environmental characteristics, including current data, sediment properties, and contaminant concentrations within the sediment and in the tissues of two epifaunal species, both from this survey and those previously reported. Previously high concentrations of polychlorinated biphenyls and polyaromatic hydrocarbons are now undetectable and concentrations of heavy metals were mostly similar or reduced compared to previous data from 2002. High within-site variance suggests localised contamination footprints from being situated within a deposition zone and/or from the erosion of anthropogenic debris. Despite the persistence of some metals (arsenic, copper and lead) at one site, our study revealed high biodiversity at all three sites (22–28 taxa per 0.25 m2). Benthic community structure was influenced by a combination of factors, including sea ice characteristics, sediment type and habitat complexity. Overall, our study clearly highlights the influence of human activities on the benthos in adjacent marine habitats. The established monitoring protocols coupling diver and remote sampling will enable regular monitoring, filling a critical need for time-series data in order to detect long-term trends and interactions with climate drivers.
Similar content being viewed by others
Introduction
Humans have affected all of Earth’s ecosystems to varying degrees (Western 2001; Halpern et al. 2008; Ellis et al. 2021). Arguably among the planet’s least human-impacted are high latitude marine ecosystems in Antarctica that are covered by ice for most of the year (Halpern et al. 2008; Aronson et al. 2011). The region’s relative remoteness and inaccessibility has resulted in limited pollution, resource extraction (minerals, fishing) and habitat fragmentation making coastal marine biological assemblages in Antarctica important bellwether systems for studies of climate-related change (Aronson et al. 2011; Dayton et al. 2019).
Yet, habitats adjacent to current and former research stations are far from pristine. Sites adjacent to some stations have been polluted by shipping, spills, accidents, intentional dumping, fuel soot, seal hunting, sled dogs, and treated and untreated sewage discharge (Lenihan et al. 1990; Kennicutt II et al. 1995, 2010; Stark et al. 2005, 2016; Palmer et al. 2021). In addition, non-point source pollutants can be introduced through increases in sediment-laden freshwater runoff and wind-blown dust, which can further deliver bound anthropogenic contaminants to the marine benthos (Griscom et al. 2004; Birch et al. 2008). Remediation of environmental damage and long-term monitoring have been identified as high priorities by The Antarctic Treaty’s Protocol on Environmental Protection (Kennicutt II et al. 2010; CEP 2021). However, ecological responses to many of these human contaminants and stressors remain poorly characterised, limiting our ability to manage and reduce human impacts in coastal areas.
Seventy-six research stations are operational in Antarctica, with the majority of these within coastal areas (COMNAP 2017). One of the largest is the United States’ McMurdo Station (MCM) located on Hut Point Peninsula, which houses > 1000 people in the summer. Owing to its size and diverse range of activities including scientific research and related logistical support (shipping, aviation, ground vehicles, fuel storage, power generation, construction, solid waste processing, sewage treatment, etc.), coastal marine soft-sediment benthic habitats near MCM in Winter Quarters Bay have been heavily polluted (e.g., Kennicutt II et al. 1995; Negri et al. 2004, 2006). There, concentrations of hydrocarbons are comparable to those in some of the world’s busiest and most contaminated shipping ports (Lenihan 1992).
The nearest research station to MCM in terms of geographical distance is New Zealand’s Scott Base (SB). SB has a maximum summer population of ~ 80 people, a size that is more typical of the majority of research stations in Antarctica. Whilst habitats near larger stations such as MCM have been extensively monitored, much less is known about the levels of anthropogenic impact in the marine areas adjacent to smaller stations, such as SB. The only previous assessment of SB marine benthos focused on sediment contamination and showed elevated levels of hydrocarbons and heavy metals (Negri et al. 2004, 2006). Contamination was lower than at nearby MCM sites (Anderson and Chague-Goff et al. 1996), and similar to levels reported elsewhere in Antarctica (Alam et al. 1993; Giordano et al. 1999; Santos et al. 2005). However, there has been no ongoing marine benthic contaminant monitoring near SB since the early work of Negri and colleagues.
Seafloor macroinvertebrate assemblages are regularly used for environmental monitoring because many of the species are sedentary, long-lived and sensitive to changes in water and sediment quality. Changes in Antarctic macrobenthic communities have been observed in response to natural disturbances such as variations in sea ice cover (Norkko et al. 2007; Lohrer et al. 2013) and ice scour (Gutt et al. 1996), as well as local anthropogenic impacts (Dayton et al. 1970; Conlan et al. 2004; Tin et al. 2009) and global climate change (Aronson et al. 2011). In the marine environment adjacent to SB, wastewater is discharged and seafloor observations have provided evidence of past anthropogenic pollution (glass, metal, wood, vehicle parts, etc.) (Negri et al. 2006; Hale et al. 2008). Understanding the impacts of SB activities on the local marine environment is imperative, particularly as sewage and metal and chemical contaminants have been shown to modify benthic communities elsewhere in multiple locations (Stark et al. 2014; Palmer et al. 2021), reducing biodiversity, changing species abundances and altering the prevalence of dominant species (Conlan et al. 2004; Stark et al. 2014; Palmer et al. 2021).
The degree to which marine assemblages in the receiving environment are affected by contaminants can depend on many factors, including their proximity to the source, the relative sensitivities of the biota to differing concentrations and combinations, dispersion characteristics and the initial concentration of the pollutant/ contaminant. The latter two factors are considerably influenced by local currents and hydrodynamics and are therefore important elements for determining potential contamination footprints, but are currently not well characterised at SB.
The objectives of this research were to (i) compare epifaunal community composition and biodiversity at two sites close to SB and at a reference site further away, and (ii) contextualise the findings with current speed and direction data from the SB sites, elucidating contaminant transport potential and their relative vulnerabilities to anthropogenic stressors. We also (iii) compare the 2019 data to contamination information recorded previously by Negri et al. (2006) as an indication of potential improvement (or lack thereof).
Materials and methods
Site locations and descriptions
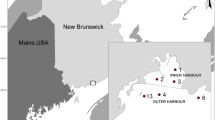
Sampling took place at three coastal marine sites around Hut Point Peninsula, southern Ross Island during October–November 2019 (Fig. 1; Table 1). Two of the sites were adjacent to SB and were chosen to coincide with potential contamination entry points from freshwater runoff and with sites previously sampled for sediment contamination (Negri et al. 2004, 2006). These sites were Scott Base 1 (SB1) on the southern coast of Pram Point approximately 300 m west-southwest of the SB sewage outfall, and Scott Base 3 (SB3) on the northern coast of Pram Point, northeast of the outfall. An initial aim was to establish up to five monitoring sites at increasing distances away from Scott Base. However, establishing additional sites around SB was impeded by practicalities including (1) a limited field work window, (2) very steep underwater terrain and thus limited seafloor area in the targeted 15–25 m depth zone, (3) sea ice conditions in front of Scott Base in 2019, including large pressure ridges directly over suitable working depths to the east of Pram Point, and (4) the Ross Ice Shelf to the north of SB which prevented the establishment of sites in that direction. Ultimately, Arrival Heights Site 1 (AH1) was chosen as a reference site. The establishment of a reference site between SB3 and AH1 was decided against due to past and recent reports of high contamination within the sediment and benthic fauna in front of MCM in Winter Quarters Bay and towards Cape Armitage (Fig. 1; Lenihan et al. 1990; Kennicutt II et al. 1995; Negri et al. 2004, 2006; Palmer et al. 2022), and thick spicule mats covering the sediments at sites near Cape Armitage that made these areas unsuitable for contaminant sampling (Klein et al. 2014).
Study area and sites sampled. A the southern half of Ross Island, with Hut Point Peninsula to the southwest. B The southern tip of Hut Point Peninsula with the site locations indicated. C Pram Point and Scott Base (green buildings), with information on summertime freshwater flows (yellow arrows) and positions of the RO intake/discharge and sewage discharge (purple arrows, with * denoting the sewage outfall). Predominant current flow directions for the two sites with ADCP current meters are also shown for reference (see methods and results for more details), and the prevailing current direction on the western side of Cape Armitage in dark blue (Littlepage 1965; Barry et al. 1988). Note that the pressure ridges were much closer to shore in 2019 than they are in this image from a prior year
Site characterisation and sampling
Two acoustic doppler current profilers (ADCP; Nortek Signature 500) were deployed at both SB sites from October to December 2019 to capture information on current speed and direction. Both ADCPs were deployed in a downward orientation and set with a 2 min sampling period in 1 m vertical depth bins from the underside of the ice to the seabed. Custom frames and tripods were used to suspend the instruments so that the instrument transducer heads were ~ 0.5 m beneath the under-surface of the ice and clear of any brash/platelet ice. Extra non-magnetic weights were added to the frames to maintain each instrument’s vertical orientation throughout the deployment.
Information on benthic habitats and epifaunal assemblage composition were collected using high resolution video. At each site, two 25 m transects were established at ~ 22 m depth. Along each transect, diver collected video was recorded from a height of 0.5 m above the seabed. Additional video footage of the seafloor surrounding each transect was taken remotely from the surface using a cabled submersible remotely operated vehicle (ROV). Multiple overlapping passes were made across the seabed transects at ~ 0.5 m depth contours between ~ 20 and 26 m in order to create a 2D orthomosaic image of each site.
To determine characteristics of surface sediments at each site, divers used sterile plastic jars to collect scrapes of surface sediment (upper 2 cm). Each replicate was a ~ 500 g composite of five individual sediment scrapes (jars). After collection, all replicates were kept in the dark and frozen until analysis. Four replicate samples were analysed for sediment contaminant concentrations (metals, PCBs, and hydrocarbons) and one composite replicate per site was analysed for particle size distribution, organic matter content, and pigment concentration (chlorophyll a and phaeophytin).
Samples of two sessile suspension-feeding taxa, the sponge Sphaerotylus antarcticus and the bivalve Laternula elliptica, were collected for tissue contaminant concentration (body burden) analyses. Data from sessile benthic organisms are likely to be more reflective of a site’s history of exposure to contaminants than analyses of mobile species (e.g., fish such as Trematomus spp., Notothenia spp.) where site fidelity is less certain. Long-lived suspension-feeding taxa living on (e.g., sponges) and in (e.g., infaunal bivalves) seafloor sediments, and that filter large volumes of water over their lifespans, make excellent bio-indicator species due to the greater probability of concentrating diffuse contaminants (de Mestre et al. 2012; Padovan et al. 2012; Batista et al. 2014; Orani et al. 2022). In addition, S. antarcticus and L. elliptica were common to abundant at all three of the sites sampled and were previously collected for contaminant analyses by Negri et al. (2006), enabling temporal comparisons. Divers attempted to collect two other sponges (Homaxinella balfourensis and Mycale sp. in addition to S. antarcticus), but both additional species were absent at one or more of the three sites and were thus not analysed. At each site, a total of four replicate samples of 20–30 g blotted tissue wet weight were collected for S. antarcticus and L. elliptica. Sponge tissue was removed in situ using a plastic knife and L. elliptica specimens were removed whole from the sediment. After collection, each sample was placed into a double ziplock bag and frozen until analysis.
Current meter processing
ADCP data were downloaded, extracted from their raw formats, and averaged into 10 min intervals. A magnetic declination of 141.09° E was applied to the measured current direction to correct the readings to reflect true north and a pressure offset was applied to standardise depths relative to ambient air pressure at the seawater surface. Data were then assessed for quality based on internal measurements of instrument tilt, signal correlation and signal strength and removed if quality thresholds were not met. Although the ADCPs were able to record for 4.5 weeks, the measuring transducers on each instrument became progressively obstructed by platelet ice growth, leading to reduced sampling range followed by complete loss of reliable data. Site SB1 produced 21 days of reliable data, with the first 17 days spanning the entire water column and the remaining 4 days recording at increasingly shallower depths. Site SB3 produced 12 days of data for the entire water column before becoming fouled.
Video analysis
Analysis of the diver-collected video was done using individual frames rather than continuous footage (Cummings et al. 2006; Thrush et al. 2011; Cummings et al. 2018). The video along each transect was ‘divided’ into 10 equal time segments and still frames were grabbed at random from the first, third, fifth, seventh and ninth segments. This ensured the independence of replicates and that the entire length of each transect was represented in the sampling. If any of the frames were of insufficient quality for analysis (e.g., blurry), a new one was selected. Eight video frames were analysed per transect (i.e., n = 8 per transect and n = 16 per site) by one individual to minimise observer bias. The still frames were imported into Adobe Photoshop software and scaled to encompass a standard field of view of 56 cm wide by 45 cm high. A computerised grid consisting of 80 equal sized squares was then superimposed onto each image. Each image was analysed in several stages. First, each of the 80 grid cells was classified as being dominated by boulder, rock, cobble, pebble, gravel, sand, shell hash, or obscured, with sizes classes following Wentworth (1922). The proportional representation of each substrate class in each image was calculated by dividing the number of cells where it was dominant by the total available cells (i.e., 80 minus the “obscured” cells). For large sessile animals (that could cover multiple grid cells in an image) and abundant small sessile animals (difficult to count individually), a technique similar to that used for the base substrate was used to estimate their presence and relative abundance. That is, the number of cells where they were present was divided by the total available cells. Most of the large taxa (mobile and sessile) in the images were able to be identified to genus or species levels, whereas smaller taxa were more difficult to identify (the maximum number of taxa identified per frame was 11). Mobile animals that were visible in the images (e.g., seastars, brittle stars, urchins, sea spiders) were counted individually rather than using the proportional representation method.
Sediment and animal tissue analysis
To determine sediment characteristics, sediment samples were analysed for particle size distribution, organic matter content, and pigment concentrations using previously published standard methods (e.g., Lohrer et al. 2004) within one month of collection. Samples for particle size distribution were immersed in a 9% hydrogen peroxide solution to digest any organic material and grain size fractions were determined by a wet sieving/pipetting method. Organic matter content was assessed via combustion of dry sediment (48 H at 60 °C) in a muffle furnace (5.5 h at 400 °C) and expressed as percent dry weight loss on ignition. Frozen chlorophyll a and phaeophytin content samples were freeze-dried, with pigments then extracted in ethanol and analysed spectrophotometrically (Turner Designs 10-AU) (Sartory 1982), yielding pigment concentrations.
Analysis of contaminants was conducted on sediment and animal tissue, as per Negri et al. (2006), using standard methods in an accredited laboratory in Hamilton, New Zealand. Sediments were sieved and the fraction < 2 mm retained, before being dried to a moisture content of 2–5% and mechanically ground to produce homogenous samples for analysis. Bivalve tissue was removed from the shell, and all animal tissue samples were chopped, minced and blended to produce homogenous samples. Individual tests were conducted on each sample for six metals (Arsenic (As), Cadmium (Cd), Copper (Cu), Lead (Pb), Mercury (Hg), and Zinc (Zn)) following nitric/ hydrochloric acid digestion, and detected using ICP-MS. Polycyclic aromatic hydrocarbons (PAHs) within animal tissue (n = 16; Acenaphthene, Acenaphthylene, Anthracene, Benzo[a]anthracene, Benzo[a]pyrene, Benzo[b]fluoranthene + Benzofluoranthene, Benzo[g,h,i]perylene, Benzo[k]fluoranthene, Chrysene, Dibenzo[a,h]anthracene, Fluoranthene, Fluorene, Indeno(1,2,3-c,d)pyrene, Naphthalene, Phenanthrene and Pyrene) and within the sediment (n = 20; with the additions of 1-Methylnaphthalene, 2-Methylnaphthalene, Benzo[e]pyrene and Perylene) were assessed using sonication extraction, solid-phase extraction clean up, followed by GC–MS SIM analysis. The presence of thirty-five polychlorinated biphenyl congeners (PCBs; PCB-18, PCB-28, PCB-31, PCB-44, PCB-49, PCB-52, PCB-60, PCB-77, PCB-81, PCB-86, PCB-101, PCB-105, PCB-110, PCB-114, PCB-118, PCB-121, PCB-123, PCB-126, PCB-128, PCB-138, PCB-141, PCB-149, PCB-151, PCB-153, PCB-156, PCB-157, PCB-159, PCB-167, PCB-169, PCB-170, PCB-180, PCB-189, PCB-194, PCB-206 and PCB-209) were examined using sonication extraction, solid-phase extraction clean up followed by GC–MS analysis. Sediments were additionally analysed for seven petroleum hydrocarbons (C7-C9, C10-C11, C12-C14, C15-C20, C21-C25, C26-C29 and C30-C44) using sonication extraction, silica clean up and GC-FID analysis, and animal tissues were additionally analysed for total lipid content using gravimetric analysis.
Statistical analyses
The seafloor biodiversity data from SB1, SB3 and AH1 were compiled into a sample-by-taxon matrix and imported into PRIMER (version 7) software for analysis. Differences in seafloor community composition were assessed from Bray–Curtis similarities following a fourth-root transformation of the raw abundance data (to down-weight the influence of abundant taxa on the results). Ordination plots were created (non-metric multidimensional scaling, nMDS) and tests for significant differences in community composition among sites were conducted (Permutational Multivariate Analysis of Variance, PERMANOVA, with “Site” as a fixed factor in post-hoc tests; Similarity Percent, SIMPER). Environmental data collected at each site were also compiled and used to describe differences among sites (e.g., Principal Components Analyses, PCA) (Clarke 1993; Clarke et al. 2006).
Results
Site characteristics
There were differences in sea ice characteristics between sites (Table 1). The sea ice was thickest at SB3 and thinnest at AH1 (Table 1). AH1 had almost no snow above the sea ice and almost no platelet/brash ice underneath (Table 1), resulting in higher underwater light availability.
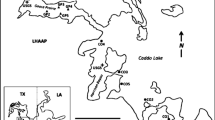
Orthomosaic maps of the seafloor at each site produced from composite images from the ROV (Fig. 2), also revealed differences between sites. The largest differences were observed between the reference site AH1 and the two SB sites (SB1, SB3). The AH1 seabed was dominated by cobbly volcanic rock interspersed with scoria rubble and gravel and few large boulders. Anthropogenic debris was found within the site, consisting of old rope and floats and a gangplank 300 m south of the transect which fell off a ship in 1960 (Dayton et al. 2016). The two SB sites were dominated by a mixture of moderately unconsolidated volcanic scoria rubble and gravels with interspersed rocky outcrops and were steeply sloped (~ 40° compared to ~ 30° at AH1). Both sites contained bivalve shell hash, predominantly from the infaunal bivalve, Limatula hodgsoni, and high cover of an unidentified filamentous ‘fluffy’ turf, which was likely comprised in part from the silica spicules of sponges. SB3 had larger patches of sediment and slightly more ambient light than SB1 (diver obs.). The biggest difference between the two SB sites was the large amount of anthropogenic debris occurring at SB3. This included glass bottles, bamboo flag poles, rusting metal drums, wooden boards, and food waste (corn cobs). Rusting and disintegrating metal turned the sediment an orange/red colour in patches at SB3.
Orthomosaic of one 25 m transect at a Arrival Heights (AH1), c Scott Base 1 (SB1) and e Scott Base 3 (SB3), along the 22 m depth contour. Images b, d, and f shows an enlarged image from each transect at AH1, SB1 and SB3, respectively. The top of each image is towards the shore, and because of steep sloping of the seabed, is slightly shallower than the bottom of the image
Sediment characteristics also differed by site (Table 2). For example, sediment mud content was 5–7 times higher at SB3 (62%) compared to the other two sites (9–12%). Sediment organic matter content, which is often positively correlated with mud content, was highest at SB3 (Table 2). Sediment chlorophyll a and phaeophytin content were up to 10 times higher in sediments at AH1 relative to SB1 and SB3 (Table 2).
Current strength and direction
Hut Point Peninsula, where two ADCPs were deployed, has diurnal tides with a range of ~ 1 m on a spring tide. Currents at SB1 were relatively weak (median and mean < 6 cm/s; Online Resource 1) and uniform from the surface to the seabed (Fig. 3C). However, brief pulses of strong flow were recorded (18–20 cm/s). An oscillating eastward-westward flow regime was observed (Fig. 3A), though the easterly currents were generally stronger than the westerly currents, suggesting the predominant residual flow pattern is from SB1 towards the SB wastewater discharge point (Fig. 1). Currents at SB3 did not follow a bi-directionally pattern but were instead predominately in a south-westerly direction. Median, mean and near maximum currents were weaker, on average, than those at SB1, with bottom water currents almost half of those at SB1 (Online Resource 2). Additionally, a more vertical current structure was observed at SB3, with currents faster underneath the ice, and slower near the seafloor (Fig. 3D).
Current speed, direction and surface to depth profiles of current flow over time at SB1 (A, C) and SB3 (B, D). A and B the distribution of depth-averaged current direction (degrees True) and velocity (m/s) over the full deployment period. The compass rose indicates the percentage of time when currents are flowing in a given direction at a given speed. C and D surface to depth profiles of current flow speed over time
Contaminant concentrations within the sediment
With very few exceptions, concentrations of all PAH and PCB congeners in sediments at all sites were below the detection thresholds of the analytical procedures used. Total PAH and PCB concentrations (i.e., all congeners combined) were also below detection thresholds at all sites, indicating very little existing organic contamination at the study sites in 2019. The only exception was petroleum hydrocarbons, which, while below detection limits at SB1 and AH1 (< 70 mg/kg dry weight), were present in moderate concentrations at SB3 (average 157.5 ± 52.2, range 90–300 mg/kg dry weight).
Heavy metals were detected in the sediments at all three sampling sites (Fig. 4, Online resource 3). Average concentrations of As, Cu, Pb, Zn, and Cd were generally highest at SB3. In contrast, the concentration of Hg was highest at AH1, with all replicates at this site exceeding the indicative sediment toxicity Default Guideline Value for Hg of 0.15 mg/kg dry weight (Fig. 4).
Average (+ 1 SE) sediment and animal tissue heavy metal contaminant concentrations at SB1, SB3 and AH1. The horizontal black line indicates the sediment toxicity default guideline values (DGV, developed by ANZECC and ARMCANZ (2000)) which “indicate the concentrations below which there is a low risk of unacceptable effects occurring, and should be used, with other lines of evidence, to protect aquatic ecosystems”. DGVs for Zn and Cd are off scale and therefore not shown. N.B. the difference in scale on the y-axis between sediment and tissue data, and Cd and Pb are in a different order for sediment and tissue concentrations to allow for the lower concentrations to be visible
Considerable among-replicate variation was detected at SB3. One of the four replicates had substantially higher concentrations of all metal species tested, with concentrations of some metals at (Cu) or above (As, Pb) indicative sediment toxicity guideline values (ANZECC & ARMCANZ 2000). The Pb concentration in this sample was 100 times higher than that of the other samples at the site, while As was ~ 10 times higher, and Cu, Cd and Hg were ~ 3 times higher.
Contaminant concentrations in animal tissues
Similar to the sediment analysis, concentrations of PAHs and PCBs within animal tissues were generally below detection limits. While some of the individual PCB congeners (PCB-52, PCB-101, PCB-118, PCB-138, PCB-149 and PCB-153) were just above the detection threshold in L. elliptica tissues sampled at AH1, the concentrations were still very low, with total PCBs < 0.02 mg/kg tissue in all replicates of both species at all three sites.
Heavy metal contaminants were detected in the tissues of both suspension feeding species (Online resource 3, Fig. 4). Cd, which was in very low concentrations in sediment, was relatively concentrated in the tissues of both species (Online resource 3). Across all metal species, concentrations in L. elliptica tended to be highest at SB1, intermediate at SB3, and lowest at AH1 (Fig. 4). No site-related pattern was apparent for S. antarcticus.
Epifaunal community structure
The ecological community data gathered from frame grabs of diver-collected video revealed all sites had high diversity, with a total of 22–28 taxa per site (see Online Resource 4 for all species and abundances). Although 28 individual taxa were recorded at SB3, the site with the highest average richness, evenness and diversity of taxa per frame was SB1. AH1 had the lowest average richness, abundance and diversity per frame (Table 3).
There were distinct differences in community assemblages between all sites (Pperm < 0.001 for all pairwise contrasts, PERMANOVA; Fig. 5), however, the two SB sites were more similar to each other (31% similarity) than either one was to AH1 (13 and 17% similarity, SIMPER; Table 4). The two SB sites were characterised by relatively high abundances of the brittle star Ophiacantha antarctica, cone sponges Polymastia invaginata, and sea spiders (Pycnogonida), with the stoloniferous soft coral Clavularia frankliniana being relatively rare. Comparatively, C. frankliniana was the most abundant epifaunal taxon observed at the AH1 site, whilst O. antarctica, P. invaginata and pycnogonids were rare to absent (absent, 16th, and 20th most abundant, respectively).
Discussion
Marine benthic habitats adjacent to Antarctic research stations have the potential to be highly impacted. Heavy metals, PAHs and PCBs were detected in sediments and in animal tissues in the vicinity of SB in 2002 (Negri et al. 2006), presumably the consequence of base activities in years prior. Base practices, including sewage treatment and pollution management, have improved substantially since 2002, and contaminant levels within animal tissues were found to be much lower in 2019. For example, PAHs and PCBs were below detection limits in 2019, and sediment heavy metal concentrations were mostly present at similar or lower levels compared to 2002, except for an increase in some metal species (As and Pb) at SB3. However, to our knowledge, there have been no assessments of seafloor community characteristics and diversity until now. Our study revealed diverse benthic communities at 22 m depth at all three sites (a total of 22–28 taxa per sample per site), including SB3, which was once a dump site and which current flows and grain size indicate may be a contaminant depositional zone.
The benthic communities at both SB sites were considerably different to AH1, being characterised by relatively high abundances of the brittle star O. antarctica, cone sponges P. invaginata, and sea spiders (Pycnogonida). Comparatively, these species were rare to absent at AH1, and instead the soft coral C. frankliniana was the most abundant epifaunal taxon. Differences in benthic community composition between sites can be partially attributed to variation in benthic substates and habitats between the three sites, as highlighted by the orthomosaic maps (Fig. 2). For example, the two SB sites had much steeper slopes and were dominated by a mixture of unconsolidated volcanic scoria rubble compared to cobbly volcanic rock at AH1. Differences and changes to seafloor complexity (both abiotic and biotic) can influence macrobenthic communities in multiple ways. The mechanisms may involve reducing the erosion of fine sediment from the bed, creating biogenic habitat for encrusting organisms, and providing protection to other organisms against predation (Thrush et al. 2013; Smith et al. 2015).
Analysis of the surface sediment further differentiated the three sites, especially between the two SB sites. Sediments at SB3 had high proportions of silt and clay, with 63% mud content compared to < 12% at the other two sites (Table 2). High rates of suspended fine sediment deposition leading to elevated muddiness are likely to influence benthic communities both directly (e.g., by smothering small organisms and interfering with their food gathering) and indirectly (e.g., by altering near-bed oxygen, ammonium, and sulphide concentrations and sediment biogeochemistry) (Ellis et al. 2002; Marinelli et al. 2002; Lohrer et al. 2004; Cummings et al. 2009). When coupled with current data, the high sediment mud content and organic enrichment observed at SB3 is unlikely to have come from the sewage outfall. The large-scale circulation pattern in McMurdo Sound moves water southward past Cape Evans towards Cape Armitage, whereupon it turns east north-east after Cape Armitage to flow under the Ross Ice Shelf (Robinson et al. 2012), and would result in SB3 largely being in the lee of the prevailing north-easterly flow and potentially in a recirculation eddy. This and the weaker currents recorded at SB3, especially near the sea floor, suggest that it may be a fine sediment deposition zone, which is further supported by the high proportion of silt and clay.
The deposition of silt and clays can additionally alter benthic macrofaunal communities via the transportation of bound contaminants (Affleck et al. 2014). Previous determination of contamination by Negri et al. (2004; 2006) found evidence of sediment and animal tissue contamination at both SB sites including PAHs, PCBs and metals (Cu, Cd, Zn, Pb and As). Our assessment at three sites in 2019 revealed PAHs and PCBs are now below detection limits or present in very low concentrations, however, the presence of metals was evident at all sites (Fig. 4). Within the sediment, metal concentrations have decreased at SB1 compared to Negri et al. (2006), but are the same (Cu, Cd, Hg), decreased (Zn) or increased by at least an order of magnitude (Pb and As) at SB3 (Online resource 3). These sediment contaminant concentrations at SB are comparable to those reported adjacent to similar sized research stations and much lower than those reported at nearby MCM sediments (e.g., Lenihan 1992; Kennicutt II et al. 1995; Stark et al. 2003; Santos et al. 2005; Kennicutt II et al. 2010; Stark et al. 2014; Palmer et al. 2021). However, the increased concentration of Pb and As in SB3 sediments and the high variance between samples at this site (Online resource 3) suggests, similar to other studies, the differential deposition of contaminant bound sediments and/or the degradation of anthropogenic debris in situ can result in persistent and patchy contamination hot spots (Kennicutt II 2003; Negri et al. 2006).
Contaminants can be harmful to marine organisms and communities, causing pathological and molecular abnormalities (Evans et al. 2000; Corbett et al. 2014), affecting behaviour and survival (Lenihan 1992), and ultimately have been linked to reduced species diversity (Lenihan et al. 1995). However, a reduction in the accumulation of metals within the tissues of long-lived benthic species was observed at both SB1 and SB3 compared to Negri et al. (2006). These reductions in bioaccumulation along with the high diversity of benthic communities observed at both sites suggest that the current contamination impact is likely to be low and localised around Scott Base, and that Antarctic benthic communities may have a level of resilience to moderate levels of pollution. Whilst more extensive monitoring (including benthic infauna) is needed to explore this, high benthic abundance and diversity (especially of sponges and bryozoans) has recently been recorded close to MCM despite its level of pollution (Dayton et al. 2019).
Benthic community structure is influenced by a complex interaction of multiple biotic and abiotic factors, such as sea ice characteristics, current flows, sediment type and food availability, as well as species interactions (Dayton et al. 1969; Gutt et al. 1996; Raguá-Gil et al. 2004; Cummings et al. 2018). Differences in land-fast sea ice characteristics were particularly evident within this study and may explain the distinct differences in benthic communities between both SB sites and AH1 (Fig. 5, Table 4; Clark et al. 2013). Thicker sea ice, as observed at both SB sites, attenuates more light, reducing microalgal abundance, as shown by the low sediment chlorophyll a concentrations (< 2 µg/g), and can result in currents providing the dominant food source to benthic communities (Dayton et al. 1986; Norkko et al. 2007; Kim et al. 2019). Comparatively, AH1 was characterised by thinner ice cover, greater light availability and thus sediment chlorophyll a concentrations that were 20–40 times higher (28 µg/g). Food supply is known to affect biodiversity in nearshore Antarctic communities (Norkko et al. 2007; Thrush et al. 2011; Lohrer et al. 2013) and therefore is likely to be a significant factor in the dissimilarity observed in benthic communities between AH1 and the two SB sites. Altered food and climate patterns are capable of driving relatively rapid change at the scale of the entire McMurdo Sound (Dayton et al. 2016; 2019), with AH1 being one of the sites exhibiting remarkable change since 1967. However, as a reference site to understand the anthropogenic impacts around SB, AH1 was not ideal. AH1 was on the opposite flank of Hut Point Peninsula from the SB sites, increasing the possibility of different water masses flowing past these sites, increasing their physical separation, and resulting in different levels of food supply and lower ecological connectivity (for example, dissimilar supply and limited exchange of larvae). Differences in food availability have previously been reported along the eastern and western coasts of McMurdo Sound (Barry et al. 1988; Cummings et al. 2003), attributed to proximity and connectedness to productive open water and sea ice thickness. Whilst the establishment of a reference site closer to SB than AH1 was initially confounded by accessibility and known contamination in front of MCM, future exploration of more suitable control sites can now be supported by additional knowledge of current patterns (Fig. 3) and proven utilisation of the ROV which could be used for preliminary site investigations.
Our study clearly highlights the influence of SB activities over past years on the benthos and sediments in the adjacent marine area. The sites we have established and sampled (SB1 and SB3), in combination with the historical information available from the 2002 investigations by Negri et al. (2004; 2006), are a valuable basis on which to monitor and detect future changes, and particularly those associated with the current Scott Base rebuild (ANZ 2023). Additionally, the orthomosaic images generated from the ROV footage will enable detailed comparisons of the wider area surveyed over time (Piazza et al. 2019). The infrastructure and protocols we have established will enable regular routine monitoring at future dates using combinations of diver-collected samples and remotely operated cameras, filling a critical need for time-series data gathering for detecting long-term trends and interactions with climate drivers.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon request.
References
Affleck RT, Carr M, Elliot L, Chan C, Knuth M (2014) Pollutant concentration in runoff at Mcmurdo Station, Antarctica. ERDC/CRREL Technical Report, US Army Research Engineering and Development Center, Hanover
Alam IA, Sadiq M (1993) Metal concentrations in antarctic sediment samples collected during the trans-antarctica 1990 expedition. Mar Pollut Bull 26(9):523–527. https://doi.org/10.1016/0025-326X(93)90472-V
Anderson B, Chagué-Goff C (1996) Benthic foraminifera and trace metals in sediments off the scott base sewer outfall, antarctica. Victoria University of Wellington, Wellington
Antarctica New Zealand (2023) Scott base redevelopment. https://www.scottbaseredevelopment.govt.nz/
Aronson RB, Thatje S, Mcclintock JB, Hughes KA (2011) Anthropogenic impacts on marine ecosystems in Antarctica. Ann N Y Acad Sci 1223(1):82–107. https://doi.org/10.1111/j.1749-6632.2010.05926.x
Australian and New Zealand Environment and Conservation Council (Anzecc), Agriculture and Resource Management Council of Australia and New Zealand (Armcanz) (2000) National water quality management strategy paper no. 4. https://www.waterquality.gov.au/anz-guidelines/resources/previous-guidelines/anzecc-armcanz-2000
Barry JP, Dayton PK (1988) Current patterns in Mcmurdo Sound, Antarctica and their relationship to local biotic communities. Polar Biol 8(5):367–376. https://doi.org/10.1007/BF00442028
Batista D, Muricy G, Rocha RC, Miekeley NF (2014) Marine sponges with contrasting life histories can be complementary biomonitors of heavy metal pollution in coastal ecosystems. Environ Sci Pollut Res 21(9):5785–5794. https://doi.org/10.1007/s11356-014-2530-7
Birch GF, Olmos MA (2008) Sediment-bound heavy metals as indicators of human influence and biological risk in coastal water bodies. ICES J Mar Sci 65(8):1407–1413. https://doi.org/10.1093/icesjms/fsn139
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18(1):117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Clarke K, Gorley R (2006) Primer version 6: user manual/tutorial. PRIMER-R, Plymouth
Clark GF, Stark JS, Johnston EL, Runcie JW, Goldsworthy PM, Raymond B, Riddle MJ (2013) Light-driven tipping points in polar ecosystems. Global Change Biol 19(12):3749–3761. https://doi.org/10.1111/gcb.12337
Committee for Environmental Protection. (2021). Committee for environmental protection five-year work plan. Report 23. Appendix 1. https://www.ats.aq/devAS/CEP/CEPReports?lang=e
Conlan KE, Kim SL, Lenihan HS, Oliver JS (2004) Benthic changes during 10 years of organic enrichment by Mcmurdo Station, Antarctica. Mar Pollut Bull 49(1):43–60. https://doi.org/10.1016/j.marpolbul.2004.01.007
Corbett PA, King CK, Stark JS, Mondon JA (2014) Direct evidence of histopathological impacts of wastewater discharge on resident Antarctic fish (Trematomus bernacchii) at Davis station, East Antarctica. Mar Pollut Bull 87(1–2):48–56. https://doi.org/10.1016/j.marpolbul.2014.08.012
Council of Managers of National Antarctic Programs. (2017). Antarctic station catalogue, pp. 86
Cummings V, Thrush S, Andrew NL, Norkko A, Funnell G, Budd R, Gibbs M, Hewitt JE, Mercer S, Marriott PM, Anderson O (2003) Final research report for ministry of fisheries research project ZBD2002/01 Objectives 1 & 2. Ecology and biodiversity of coastal benthic communities in Mcmurdo Sound, Ross Sea: emerging results. National Institute of Water and Atmospheric Research, Auckland
Cummings V, Thrush S, Norkko A, Andrew N, Hewitt J, Funnell G, Schwarz A (2006) Accounting for local scale variability in macrobenthos in developing assessments of latitudinal trends in the south western ross sea. Antarct Sci 18:633–644
Cummings V, Vopel K, Thrush S (2009) Terrigenous deposits in coastal marine habitats: influences on sediment geochemistry and behaviour of post-settlement bivalves. Mar Ecol Prog Ser 383:173–185
Cummings VJ, Hewitt JE, Thrush SF, Marriott PM, Halliday NJ, Norkko A (2018) Linking ross sea coastal benthic communities to environmental conditions: documenting baselines in a spatially variable and changing world. Front Mar Sci. https://doi.org/10.3389/fmars.2018.00232
Dayton PK, Robilliard GA, Devries AL (1969) Anchor ice formation in Mcmurdo Sound, Antarctica, and its biological effects. Science 163(3864):273–274. https://doi.org/10.1126/science.163.3864.273
Dayton P, Robilliard GA, Paine R (1970) Benthic faunal zonation as a result of anchor ice at Mcmurdo Sound, Antarctica. Antarct Ecol 1:244–258
Dayton PK, Watson D, Palmisano A, Barry JP, Oliver JS, Rivera D (1986) Distribution patterns of benthic microalgal standing stock at Mcmurdo Sound, Antarctica. Polar Biol 6(4):207–213. https://doi.org/10.1007/BF00443397
Dayton P, Jarrell S, Kim S, Thrush S, Hammerstrom K, Slattery M, Parnell E (2016) Surprising episodic recruitment and growth of Antarctic sponges: implications for ecological resilience. J Exp Mar Biol Ecol 482:38–55. https://doi.org/10.1016/j.jembe.2016.05.001
Dayton PK, Jarrell SC, Kim S, Parnell P, Thrush SF, Hammerstrom K, Leichter JJ (2019) Benthic responses to an Antarctic regime shift: food particle size and recruitment biology. Ecol Appl 29(1):e01823
De Mestre C, Maher W, Roberts D, Broad A, Krikowa F, Davis AR (2012) Sponges as sentinels: patterns of spatial and intra-individual variation in trace metal concentration. Mar Pollut Bull 64(1):80–89. https://doi.org/10.1016/j.marpolbul.2011.10.020
Ellis J, Cummings V, Hewitt J, Thrush S, Norkko A (2002) Determining effects of suspended sediment on condition of a suspension feeding bivalve (Atrina zelandica): results of a survey, a laboratory experiment and a field transplant experiment. J Exp Mar Biol Ecol 267(2):147–174. https://doi.org/10.1016/S0022-0981(01)00355-0
Ellis EC, Gauthier N, Klein Goldewijk K, Bliege Bird R, Boivin N, Díaz S, Fuller DQ, Gill JL, Kaplan JO, Kingston N, Locke H, Mcmichael CNH, Ranco D, Rick TC, Shaw MR, Stephens L, Svenning JC, Watson JEM (2021) People have shaped most of terrestrial nature for at least 12,000 years. Proc Natl Acad Sci 118(17):e2023483118. https://doi.org/10.1073/pnas.2023483118
Evans CW, Hills JM, Dickson JMJ (2000) Heavy metal pollution in Antarctica: a molecular ecotoxicological approach to exposure assessment. J Fish Biol 57:8–19. https://doi.org/10.1111/j.1095-8649.2000.tb02241.x
Giordano R, Lombardi G, Ciaralli L, Beccaloni E, Sepe A, Ciprotti M, Costantini S (1999) Major and trace elements in sediments from terra nova bay, Antarctica. Sci Total Environ 227(1):29–40. https://doi.org/10.1016/S0048-9697(98)00402-1
Griscom SB, Fisher NS (2004) Bioavailability of sediment-bound metals to marine bivalve molluscs: an overview. Estuaries 27(5):826–838. https://doi.org/10.1007/BF02912044
Gutt J, Starmans A, Dieckmann G (1996) Impact of iceberg scouring on polar benthic habitats. Mar Ecol Prog Ser 137:311–316. https://doi.org/10.3354/meps137311
Hale RC, Kim SL, Harvey E, La Guardia MJ, Mainor TM, Bush EO, Jacobs EM (2008) Antarctic research bases: local sources of polybrominated diphenyl ether (pbde) flame retardants. Environ Sci Technol 42(5):1452–1457. https://doi.org/10.1021/es702547a
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319(5865):948–952. https://doi.org/10.1126/science.1149345
Kennicutt Ii MC (2003) Spatial and temporal scales of human disturbance Mcmurdo Station, Antarctica. Marine Science Institute, The University of Texas, Austin, p 184
Kennicutt Ii MC, Mcdonald SJ, Sericano JL, Boothe P, Oliver J, Safe S, Presley BJ, Liu H, Wolfe D, Wade TL, Crockett A, Bockus D (1995) Human contamination of the marine environment-arthur harbor and Mcmurdo Sound, Antarctica. Environ Sci Technol 29(5):1279–1287. https://doi.org/10.1021/es00005a600
Kennicutt Ii MC, Klein A, Montagna P, Sweet S, Wade T, Palmer T, Sericano J, Denoux G (2010) Temporal and spatial patterns of anthropogenic disturbance at Mcmurdo Station, Antarctica. Environ Res Lett 5(3):034010. https://doi.org/10.1088/1748-9326/5/3/034010
Kim S, Hammerstrom K, Dayton P (2019) Epifaunal community response to iceberg-mediated environmental change in Mcmurdo Sound, Antarctica. Mar Ecol Prog Ser 613:1–14. https://doi.org/10.3354/meps12899
Klein AG, Sweet ST, Kennicutt MC, Wade TL, Palmer T, Montagna P (2014) Human impacts on the environment at high latitudes: fate, effect, and transport of contaminants near an antarctic base. Final Report (Phase 9) prepared by the Texas A&M Research Foundation for the DOD-Army-COE-Engineer Research and Development Centre, Cold Regions R&E Laboratory, Issue 52
Lenihan HS (1992) Benthic marine pollution around Mcmurdo Station, Antarctica: a summary of findings. Mar Pollut Bull 25(9):318–323. https://doi.org/10.1016/0025-326X(92)90689-4
Lenihan HS, Oliver JS (1995) Anthropogenic and natural disturbances to marine benthic communities in Antarctica. Ecol Appl 5(2):311–326. https://doi.org/10.2307/1942024
Lenihan HS, Oliver JS, Oakden JM, Stephenson MD (1990) Intense and localized benthic marine pollution around Mcmurdo Station, Antarctica. Mar Pollut Bull 21(9):422–430. https://doi.org/10.1016/0025-326X(90)90761-V
Littlepage JL (1965) Oceanographic investigations in Mcmurdo Sound, Antarctica. Biology of the Antarctic Seas II. Wiley, Hoboken, pp 1–37
Lohrer AM, Thrush SF, Hewitt JE, Berkenbusch K, Ahrens M, Cummings VJ (2004) Terrestrially derived sediment: response of marine macrobenthic communities to thin terrigenous deposits. Mar Ecol Prog Ser 273:121–138
Lohrer AM, Cummings VJ, Thrush SF (2013) Altered sea ice thickness and permanence affects benthic ecosystem functioning in coastal antarctica. Ecosystems 16(2):224–236. https://doi.org/10.1007/s10021-012-9610-7
Marinelli RL, Woodin SA (2002) Experimental evidence for linkages between infaunal recruitment, disturbance and sediment surface chemistry. Limnol Oceanogr 47(1):221–229. https://doi.org/10.4319/lo.2002.47.1.0221
Negri AP, Hales LT, Battershill C, Wolff C, Webster NS (2004) Tbt contamination identified in Antarctic marine sediments. Mar Pollut Bull 48(11):1142–1144. https://doi.org/10.1016/j.marpolbul.2004.03.004
Negri A, Burns K, Boyle S, Brinkman D, Webster N (2006) Contamination in sediments, bivalves and sponges of Mcmurdo Sound, Antarctica. Environ Pollut 143(3):456–467. https://doi.org/10.1016/j.envpol.2005.12.005
Norkko A, Thrush SF, Cummings VJ, Gibbs MM, Andrew NL, Norkko J, Schwarz AM (2007) Trophic structure of coastal Antarctic food webs associated with changes in sea ice and food supply. Ecology 88(11):2810–2820. https://doi.org/10.1890/06-1396.1
Orani AM, Vassileva E, Thomas OP (2022) Marine sponges as coastal bioindicators of rare earth elements bioaccumulation in the French Mediterranean Sea. Environ Pollut 304:119172. https://doi.org/10.1016/j.envpol.2022.119172
Padovan A, Munksgaard N, Alvarez B, Mcguinness K, Parry D, Gibb K (2012) Trace metal concentrations in the tropical sponge Spheciospongia vagabunda at a sewage outfall: synchrotron x-ray imaging reveals the micron-scale distribution of accumulated metals. Hydrobiologia 687(1):275–288. https://doi.org/10.1007/s10750-011-0916-9
Palmer TA, Klein AG, Sweet ST, Montagna PA, Hyde LJ, Sericano J, Wade TL, Kennicutt MC, Beseres Pollack J (2021) Long-term changes in contamination and macrobenthic communities adjacent to Mcmurdo Station, Antarctica. Sci Total Environ 764:142798. https://doi.org/10.1016/j.scitotenv.2020.142798
Palmer TA, Klein AG, Sweet ST, Frazier AJ, Montagna PA, Wade TL, Beseres Pollack J (2022) Using epibenthic fauna as biomonitors of local marine contamination adjacent to Mcmurdo Station, Antarctica. Mar Pollut Bull 178:113621. https://doi.org/10.1016/j.marpolbul.2022.113621
Piazza P, Cummings V, Guzzi A, Hawes I, Lohrer A, Marini S, Marriott P, Menna F, Nocerino E, Peirano A, Kim S, Schiaparelli S (2019) Underwater photogrammetry in Antarctica: long-term observations in benthic ecosystems and legacy data rescue. Polar Biol 42(6):1061–1079. https://doi.org/10.1007/s00300-019-02480-w
Raguá-Gil JM, Gutt J, Clarke A, Arntz WE (2004) Antarctic shallow-water mega-epibenthos: shaped by circumpolar dispersion or local conditions? Mar Biol 144(5):829–839. https://doi.org/10.1007/s00227-003-1269-3
Robinson NJ, Williams MJM (2012) Iceberg-induced changes to polynya operation and regional oceanography in the Southern Ross Sea, Antarctica, from in situ observations. Antarct Sci 24(5):514–526. https://doi.org/10.1017/S0954102012000296
Santos IR, Silva-Filho EV, Schaefer CEGR, Albuquerque-Filho MR, Campos LS (2005) Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Mar Pollut Bull 50(2):185–194. https://doi.org/10.1016/j.marpolbul.2004.10.009
Sartory DP (1982) Spectrophotometric analysis of chlorophyll a in freshwater phytoplankton. Department of Environment Affairs, Hydrological Research Institute, Pretoria, p 163
Smith J, O’brien PE, Stark JS, Johnstone GJ, Riddle MJ (2015) Integrating multibeam sonar and underwater video data to map benthic habitats in an East Antarctic nearshore environment. Estuarine Coastal Shelf Sci 164:520–536. https://doi.org/10.1016/j.ecss.2015.07.036
Stark JS, Riddle MJ, Snape I, Scouller RC (2003) Human impacts in antartic marine soft-sediment assemblages: correlations between multivariate biological patterns and environmental variables at casey station. Estuar Coast Shelf Sci 56(3):717–734. https://doi.org/10.1016/S0272-7714(02)00291-3
Stark JS, Snape I, Riddle MJ, Stark SC (2005) Constraints on spatial variability in soft-sediment communities affected by contamination from an Antarctic waste disposal site. Mar Pollut Bull 50(3):276–290. https://doi.org/10.1016/j.marpolbul.2004.10.015
Stark JS, Kim SL, Oliver JS (2014) Anthropogenic disturbance and biodiversity of marine benthic communities in Antarctica: a regional comparison. PLoS ONE 9(6):e98802. https://doi.org/10.1371/journal.pone.0098802
Stark JS, Corbett PA, Dunshea G, Johnstone G, King C, Mondon JA, Power ML, Samuel A, Snape I, Riddle M (2016) The environmental impact of sewage and wastewater outfalls in Antarctica: an example from Davis Station, East Antarctica. Water Res 105:602–614. https://doi.org/10.1016/j.watres.2016.09.026
Thrush SF, Cummings VJ (2011) Massive icebergs, alteration in primary food resources and change in benthic communities at Cape Evans, Antarctica. Mar Ecol 32(3):289–299. https://doi.org/10.1111/j.1439-0485.2011.00462.x
Thrush SF, Hewitt JE, Lohrer AM, Chiaroni LD (2013) When small changes matter: the role of cross-scale interactions between habitat and ecological connectivity in recovery. Ecol Appl 23(1):226–238. https://doi.org/10.1890/12-0793.1
Tin T, Fleming ZL, Hughes KA, Ainley DG, Convey P, Moreno CA, Pfeiffer S, Scott J, Snape I (2009) Impacts of local human activities on the Antarctic environment. Antarct Sci 21(1):3–33. https://doi.org/10.1017/S0954102009001722
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30(5):377–392
Western D (2001) Human-modified ecosystems and future evolution. Proc Natl Acad Sci 98(10):5458–5465. https://doi.org/10.1073/pnas.101093598
Acknowledgements
This work was funded by Antarctica New Zealand. Manuscript preparation was supported by the New Zealand Government's Strategic Science Investment Fund (SSIF) to the National Institute for Water & Atmospheric Research (NIWA). Thank you to Vonda Cummings for reviewing a draft of the manuscript, and two anonymous reviewers.
Author information
Authors and Affiliations
Contributions
AML was principal investigator, designed the study and compiled and analysed the data. AML, PM, RB, DB and LWT conducted the field work. PM and LWT collected and processed the video data. DB collected and BG processed the oceanographic data. LWT processed photogrammetry data. SM and AML wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lohrer, A.M., Mangan, S., Marriott, P. et al. Diverse marine benthic communities and reduced anthropogenic contaminants near Scott Base (Hut Point Peninsula, Ross Island, Antarctica). Polar Biol 46, 1039–1052 (2023). https://doi.org/10.1007/s00300-023-03181-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03181-1