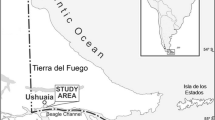
Abstract
Reproduction is a highly expensive process that during gonadal development requires an important supply of nutrients. The allocation of energy can vary throughout the reproductive cycle, between sexes and development modes. During research cruise aboard the RV Puerto Deseado in April 2016, we collected fifteen species of sea stars from the southernmost region of Argentina. The main purposes of the present study were threefold: first, to revise and report novel information on the reproductive strategies and energetic density (ED) of body components from the asteroids of the southwestern Atlantic Ocean including Burdwood Bank/ MPA Namuncurá; second, to compare the ED of these body components across species with contrasting reproductive strategies; third, to provide a tool to estimate the ED from dry mass of organs of the sea star species examined. Ovaries of Diplopteraster verrucosus (a brooder with a nidamental chamber) revealed a significantly greater ED than did the testes (29.81 ± 1.38 and 17.76 ± 1.59, respectively). In Glabraster antarctica (a broadcaster with yolky eggs and facultative planktotrophic larvae), the gonads had EDs of 25.78 ± 3.16 and 19.21 ± 0.52 (females and males, respectively). While in Peribolaster folliculatus (a broadcaster with eggs with low yolk content and inferred planktotrophic larvae) there was no significant difference in the ED values between sexes (females: 22.79 ± 1.10 and males: 20.46 ± 1.05). For the pyloric caeca, ED values did not reveal any difference between sexes, although in P. folliculatus, the ED was significantly higher than that for D. verrucosus and G. antarctica (25.90 ± 1.00, 23.03 ± 0.34, and 22.66 ± 0.65, respectively). The body wall had higher ED values in D. verrucosus and P. folliculatus than that for all the sea star species analyzed (46.48 ± 1.63, 51.17 ± 1.34, respectively). Higher ED values in the ovaries could be related to the nutrition of offspring, while differences found in the body wall may reflect the skeletal structure of this body component. This research provides basic information for understanding the differences on energetic allocation when contrasting development modes are considered.




Similar content being viewed by others
References
Almandoz GO, Hernando MP, Ferreyra GA, Schloss IR, Ferrario ME (2011) Seasonal phytoplankton dynamics in extreme southern South America (Beagle Channel, Argentina). J Sea Res 66:47–57
Arntz W, Rauschert M (2015) Antarctic macrobenthos: a feld guide of the invertebrates living at the antarctic seafloor. Arntz & Rauschert Selbstverlag, Wurster Nordseekueste
Bavington CD, Lever R, Mulloy B, Grundy MM, Page CP, Richardson NV, McKenzie JD (2004) Anti-adhesive glycoproteins in echinoderm mucus secretions. Comp Biochem Physiol B 139:607–617
Bernasconi I (1962) Asteroideos Argentinos III. Familia Odontasteridae. Rev Mus Argent Cienc Nat Bernardino Rivadavia Inst Nac Invest Cienc Nat (Argent), Zool 9(1):1–25
Bernasconi I (1970) Equinodermos antárticos. II. Asteroideos. 3. Asteroideos de la extremidad norte de la Península Antártica. Rev Mus Argent Cienc Nat Bernardino Rivadavia Inst Nac Invest Cienc Nat (Argent) Zool 9:211–281
Blake DB (2000) The class Asteroidea (Echinodermata): fossils and the base of the crown group. Am Zool 40:316–325
Bosch I (1989) Contrasting modes of reproduction in two Antarctic asteroids of the genus Porania, with the descrption of unusual feeding and non-feeding larval types. Biol Bull 177:77–82
Bosch I, Pearse J (1990) Developmental types of shallow-water asteroids of McMurdo Sound, Antarctica. Mar Biol 104:41–46
Bosch I, Slattery M (1999) Costs of extended brood protection in the Antarctic sea star, Neosmilaster georgianus (Echinodermata: Asteroidea). Mar Biol 134:449–459
Boy C, Pérez A, Fernández D, Calvo J, Morriconi E (2009) Energy allocation in relation to spawning and overwintering of a diadromous Puyen (Galaxias maculatus) population in the southernmost limit of the species distribution. Polar Biol 32:9–14
Broyer C, Koubbi P (eds) (2014) SCAR biogeographic Atlas of the Southern Ocean. Scientific Committee on Antarctic Research Cambridge, UK
Byrne M (1996) Viviparity and intragonadal cannibalism in the diminutive sea stars Patiriella vivipara and P. parvivipara (family Asterinidae). Mar Biol 125:551–567
Byrne M (2005) Viviparity in the sea star Cryptasterina hystera (Asterinidae)—conserved and modified features in reproduction and development. Biol Bull 208:81–91
Calow P (1984) Economics of ontogeny-adaptational aspects. In: Shorrocks B (ed) Evolutionary ecology. Blackwell, Oxford, pp 81–104
Clark AM, Downey ME (1992) Starfshes of the Atlantic, vol 3. Chapman & Hall, London, p 794
Clarke A (1987) Temperature, latitude and reproductive effort. Mar Ecol Prog Ser 39:89–99
Clarke A, Johnston NM (2003) Antarctic marine benthic diversity. Oceanogr Mar Biol 41:47–114
Cossi PF, Boy CC, Giménez J, Pérez AF (2015) Reproductive biology and energy allocation of the sea star Cosmasterias lurida (Echinodermata: Asteroidea) from the Beagle Channel, Tierra del Fuego, Argentina. Polar Biol 38:1321–1333
Chia FS (1974) Classification and adaptive significance of developmental patterns in marine invertebrates. Thalassia jugosl 10:121–130
Chia F, Walker CW (1991) Echinodermata: asteroidea. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates. Echinoderms and lophophorates. The Boxwood Press, Pacific Grove, vol 6, pp 301–353
De Broyer C, Danis B (2010) SCAR-MarBIN: the Antarctic marine biodiversity information network World Wide Web electronic publication. https://www.scarmarbin.be. Accessed 1 Aug 2017
De Broyer C, Clarke A, Koubbi P, Pakhomov E, Scott F, Vanden Berghe E, Danis B (eds) (2018). Register of Antarctic Marine (RAMS). http://www.marinespecies.org/rams. Accessed 22 Mar 2018
Falabella V (2017) Área Marina Protegida Namuncurá—Banco Burdwood. Contribuciones para la línea de base y el plan de manejo, Buenos Aires
Fernández DA, Lattuca ME, Boy CC, Pérez A, Ceballos SG, Vanella FA, Morriconi ER, Malanga GF, Aureliano DR, Rimbau S, Calvo J (2009) Energy density of sub-Antarctic fishes from the Beagle Channel. Fish Physiol Biochem 35(1):181–188. https://doi.org/10.1007/s10695-008-9234-1
Fieser M, Fieser LF, Toromanoff E, Hirata Y, Heymann H, Tefft M, Bhattacharya S (1956) Synthetic emulsifying agents. J Am Chem Soc 78:2825–2832
Fisher WK (1940) Asteroidea. Disc Rep 20:69–306
Fraysse CF, Clacagno JA, Pérez AF (2018) Asteroidea of the southern tip of South America, including Namuncurá Marine Protected Area at Burdwood Bank and Tierra del Fuego Province, Argentina. Polar Biol 41:2423–2433
Giese A (1966) On the biochemical constitution of some echinoderms. In: Boolootian RA (ed) Physiology of echinodermata. Interscience, New York, pp 547–576
Gillespie JM, McClintock JB (2007) Brooding in echinoderms: How can modern experimental techniques add to our historical perspective? J Exp Mar Biol Ecol 342:191–201
Hennebert E, Wattiez R, Waite JH, Flammang P (2012) Characterization of the protein fraction of the temporary adhesive secreted by the tube feet of the sea star Asterias rubens. Biofouling 28(3):289–303
Hennebert E, Leroy B, Wattiez R, Ladurner P (2015) An integrated transcriptomic and proteomic analysis of sea star epidermal secretions identifies proteins involved in defense and adhesion. J Proteom 128:83–91
Himmelman J, Lavergne Y, Cardinal A, Martel G, Jalbert P (1982) Brooding behaviour of the northern sea star Leptasterias polaris. Mar Biol 68:235–240
Horton et al (2018) World Register of Marine Species (WoRMS) WoRMS editorial board. https://www.marinespecies.org. Accessed 22 Mar, 2018
Hyman LH (1955) The invertebrates: echinodermata, the coelomate bilateria, vol 4. McGraw-Hill, New York
Jagt JW et al (2014) A starfish bed in the Middle Miocene Grand Bay Formation of Carriacou, The Grenadines (West Indies). Geol Mag 151:381–393
Jangoux M (1982) Food and feeding mechanisms: asteroidea. In: Janggoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema, Rotterdam, pp 117–157
Janosik AM, Halanych KM (2010) Unrecognized Antarctic biodiversity: a case study of the genus Odontaster (Odontasteridae; Asteroidea). Integ Comp Biol 50:981–992
Koehler R (1913) Echinodermes (astéries, ophiures et échinides): recueillis par M. Rallier du Baty, aux îles de Kerguelen, en 1913–1914. Masson
Lawrence JM (1987a) Bioenergetics of echinoderms. In: Keegan BF, O’Connor DS (eds) Echinodermata. Balkema, Rotterdam, pp 47–67
Lawrence JM (1987b) Functional biology of echinoderms. The Johns Hopkins University Press, Baltimore
Lawrence JM (1987c) Echinoderms. In: Pandian TJ, Vernberg FJ (eds) Animal energetics, vol 2. Academic Press, San Diego, pp 229–321
Lawrence JM, Guille A (1982) Organic composition of tropical, polar and temperate-water echinoderms. Comp Biochem Physiol B 72:283–287
Lawrence JM, Lane JM (1982) The utilization of nutrients by post-metamorphic echinoderms. In: Janggoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema, Rotterdam, pp 331–371
Lawrence JM, McClintock J (1994) Energy acquisition and allocation by echinoderms (Echinodermata) in polar seas: adaptations for success. In: Keegan BF, O’Connor DS (eds) Echinodermata. Balkema, Rotterdam, pp 39–52
Lawrence JM, Moran P (1992) Proximate composition and allocation of energy to body components in Acanthaster planci (Linnaeus) (Echinodermata: Asteroidea). Zool Sci 9:321–328
Lieberkind I (1920) On a starfish (Asterias groenlandica) which hatches its young in its stomach. Vidensk Medd Dan Nat hist Foren 72:121–126
Lieberkind I (1926) Ctenodiscus australis Lütken A brood-protecting asteroid. Vidensk Medd Dan Naturhist Foren 82:183–196
Lucas A (1996) Energetics of aquatic animals. Taylor & Francis, London
MacBride EW (1920) Echinoderma (part II) and Enteropneusta. Larvae of Echinoderma and Enteropneusta. Nat Hist Rep Br Antarct ‘‘Terra Nova’’ Exped 1910 Zool 3:83–94
Mah CL (2018) World Asteroidea database. https://www.marinespecies.org/asteroidea. Accessed 2018
Mah C, Neill K, Eléaume M, Foltz D (2014) New species and global revision of Hippasteria (Hippasterinae: Goniasteridae; Asteroidea; Echinodermata). Zool J Linn Soc 171:422–456
McClary DJ, Mladenov PV (1990) Brooding biology of the sea star Pteraster militaris (OF Müller): energetic and histological evidence for nutrient translocation to brooded juveniles. J Exp Mar Biol Ecol 142:183–199
McClintock J, Cameron J, Young C (1990) Biochemical and energetic composition of bathyal echinoids and an asteroid, holothuroid and crinoid from the Bahamas. Mar Biol 105:175–183
McClintock JB, Pearse JS (1987) Biochemical composition of antarctic echinoderms. Comp Biochem Physiol B 86:683–687
McClintock JB (1994) Trophic biology of Antarctic shallow-water echinoderms. Mar Ecol Prog Ser 191–202
McClintock JB, Watts SA, Marion KR, Hopkins TS (1995) Gonadal cycle, gametogenesis and energy allocation in two sympatric mid shelf sea stars with contrasting modes of reproduction. Bull Mar Sci 57:442–452
McEdward LR (1995) Evolution of pelagic direct development in the starfish Pteraster tesselatus (Asteroidea: Velatida). Biol J Linn Soc 54:299–327
McEdward LR, Miner BG (2001) Larval and life-cycle patterns in echinoderms. Canad J Zool 79(7):1125–1170
Mileikovsky SA (1971) Types of larval development in marine bottom invertebrates, their distribution and ecological significance: a re-evaluation. Mar Biol 10(3):193–213
Moore JM, Carvajal JI, Rouse GW, Wilson NG (2018) The Antarctic circumpolar current isolates and connects: Structured circumpolarity in the sea star Glabraster antarctica. Ecol Evol 8(21):10621–10633
Nance JM, Braithwaite LF (1979) The function of mucous secretions in the cushion star Pteraster tesselatus Ives. J Exp Mar Biol Ecol 40:259–266
OBIS (2018) Ocean biogeographic information system. Intergovernmental Oceanographic Commission of UNESCO. www.iobis.org, Accessed, p 2018
Paine RT (1969) The Pisaster-Tegula interaction: prey patches, predator food preference, and intertidal community structure. Ecology 50:950–961
Pastor de Ward CT, Rubilar T, Díaz-de-Vivar ME, Gonzalez-Pisani X, Zarate E, Kroeck M, Morsan E (2007) Reproductive biology of Cosmasterias lurida (Echinodermata: Asteroidea) an anthropogenically influenced substratum from Golfo Nuevo, Northern Patagonia (Argentina). Mar Biol 151(1):205
Pearse JS, Bosch I (1994) Brooding in the Antarctic: Östergren had it nearly right. Echinoderms through time. Balkema, Rotterdam, pp 111–120
Pearse JS, McClintock JB, Bosch I (1991) Reproduction of Antarctic benthic marine invertebrates: tempos, modes, and timing. Am Zool 31:65–80
Pearse JS, Mooi R, Lockhart SJ, Brandt A (2009) Brooding and species diversity in the Southern Ocean: selection for brooders or speciation within brooding clades? Smithsonian at the poles: contributions to international polar year science. Smithsonian Institute, Smithsonian Institution Scholarly Press, Washington, DC
Pérez AF, Morriconi E, Boy C, Calvo J (2008) Seasonal changes in energy allocation to somatic and reproductive body components of the common cold temperature sea urchin Loxechinus albus in a Sub-Antarctic environment. Polar Biol 31:443–449
Pérez AF, Boy C, Morriconi E, Calvo J (2010) Reproductive cycle and reproductive output of the sea urchin Loxechinus albus (Echinodermata: Echinoidea) from Beagle Channel, Tierra del Fuego, Argentina. Polar Biol 33:271–280
Pérez AF, Boy CC, Calcagno JÁ, Malanga G (2015) Reproduction and oxidative metabolism in the brooding sea star Anasterias antarctica (Lütken, 1957). J Exp Mar Biol Ecol 463:150–157
Pérez AF, Fraysse C, Boy CC, Epherra L, Javier C (2017) Reproductive biology and energetics of the brooding sea star Anasterias antarctica (Echinodermata: Asteroidea) in the Beagle Channel, Tierra del Fuego, Argentina. Rev Biol Trop 65:221–232
Piola AR, Gordon AL (1989) Intermediate waters in the southwest South Atlantic. Deep-Sea Res Oceanogr A 36:1–16
Raymond JF, Himmelman JH, Guderley HE (2004) Sex differences in biochemical composition, energy content and allocation to reproductive effort in the brooding sea star Leptasterias polaris. Mar Ecol Prog Ser 283:179–190
Raymond JF, Himmelman JH, Guderley HE (2007) Biochemical content, energy composition and reproductive effort in the broadcasting sea star Asterias vulgaris over the spawning period. J Exp Mar Biol Ecol 341:32–44
Sabatini M, Reta R, Matano R (2004) Circulation and zooplankton biomass distribution over the southern Patagonian shelf during late summer. Cont Shelf Res 24:1359–1373
Schejter L et al (2016) Namuncurá Marine Protected Area: an oceanic hot spot of benthic biodiversity at Burdwood Bank, Argentina. Polar Biol 39:2373–2386
Sladen WP (1889) Report on the Asteroidea collected by HMS Challenger. Zoology 30:893p
Sokal RR, Rohlf J (1995) Biometry. Freeman, San Francisco, CA
Stampanato S, Jangoux M (1993) Les astérides (Echinodermata) de la Baie Breid (Côte de la Princesse Ragnhild, quartier Enderby, Antarctique), avec la description d’une nouvelle espèce de Solaster. Bull Inst r Sci Nat Belg 63:1785–2184
Stilwell J, Long J (2011) Frozen in time: prehistoric life in Antarctica. Csiro Publishing, Clayton
Studer T (1885) Die Seesterne Süd-Georgiens nach der Ausbeute der deutschen Polarstation in 1882 und 1883. Nat Mus Hmb 2:141–166
Sutton M, Briggs D, Siveter DJ, Siveter DJ, Gladwell D (2005) A starfish with three-dimensionally preserved soft parts from the Silurian of England. Proc R Soc Lond B 272:1001–1006
Tablado A (1982) Asteroideos argentinos Familia Poraniidae. Comun Mus Argent Cienc Nat “Bernardino Rivadavia” Inst Nac Invest Cienc Nat (Argent). Zool 2(8):87–106
Thatje S, Steventon E (1920) Heilmayer O (2018) Energetic changes throughout early ontogeny of the brooding Antarctic sea star Rhopiella hirsuta (Koehler. Polar Biol 41(6):1297–1306
Thomson C (1876) Notice of some Peculiarities in the mode of propagation of certain Echinoderms of the Southern Sea. Zool J Linn Soc 13:55–79
Verrill AE (1914) Monograph of the shallow-water starfshes of the North Pacifc coast from the Arctic Ocean to California. Harriman Alaska Ser 14:1–408
Ward JA (1962) A further investigation of the swimming reaction of Stomphia coccinea. Am Zool 2:567–567
Acknowledgements
We are grateful to the crew of the RV “Puerto Deseado” for support during the sampling procedures as well as to Drs. Daniel Roccatagliata and Laura Schejter for their commitment to coordinating the work on board. We also thank the members of Laboratorio de Ecología, Fisiología y Evolución de Organismos Acuáticos (CADIC-CONICET) and Lic. Yamila Becker, Dr. Pablo Di Salvatore, Lic. Sebastian Franzese, and Lic. Virginia García Alonso for their technical assistance during the research cruise. We thank Dr. Gustavo Lovrich, Dr. Isidro Bosch, Dr. J. B. McClintock, and Charlotte Regula-Whitefield for reviewing the manuscript and for their valuable suggestions that have considerably improved the final version. We finally thank Dr. Gustavo Lovrich and James McClintock for their help revising the language.
Funding
This study was funded by Fundación Felipe Fiorellino, Universidad Maimónides. This is the scientific contribution 22 of the Marine Protected Area Namuncurá (National Law 26875).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All permissions for collecting samples from the 'Marine Protected Area Namuncurá (MPAN) were issued by its Administrative Council by authorizing and funding the scientific missions. All sampling procedures and experimental manipulations follow the guidelines approved by the Universidad de Buenos Aires (Facultad de Ciencias Exactas y Naturales, Bioterio Central,https://exactas.uba.ar/cicual/).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fraysse, C.P., Pérez, A.F., Calcagno, J.A. et al. Energetics and development modes of Asteroidea (Echinodermata) from the Southwestern Atlantic Ocean including Burdwood Bank/MPA Namuncurá. Polar Biol 43, 175–186 (2020). https://doi.org/10.1007/s00300-020-02621-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02621-6