Abstract
The condition and survival of Antarctic krill (Euphausia superba) strongly depends on sea ice conditions during winter. How krill utilize sea ice depends on several factors such as region and developmental stage. A comprehensive understanding of sea ice habitat use by krill, however, remains largely unknown. The aim of this study was to improve the understanding of the krill’s interaction with the sea ice habitat during winter/early spring by conducting large-scale sampling of the ice–water interface (0–2 m) and comparing the size and developmental stage composition of krill with the pelagic population (0–500 m). Results show that the population in the northern Weddell Sea consisted mainly of krill that were <1 year old (age class 0; AC0), and that it was comprised of multiple cohorts. Size per developmental stage differed spatially, indicating that the krill likely were advected from various origins. The size distribution of krill differed between the two depth strata sampled. Larval stages with a relatively small size (mean 7–8 mm) dominated the upper two metre layer of the water column, while larger larvae and AC0 juveniles (mean 14–15 mm) were proportionally more abundant in the 0- to 500-m stratum. Our results show that, as krill mature, their vertical distribution and utilization of the sea ice appear to change gradually. This could be the result of changes in physiology and/or behaviour, as, e.g., the krill’s energy demand and swimming capacity increase with size and age. The degree of sea ice association will have an effect on large-scale spatial distribution patterns of AC0 krill and on predictions of the consequences of sea ice decline on their survival over winter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During winter, a large part of Antarctic krill’s (Euphausia superba) habitat is ice-covered (Meyer et al. 2002a). At the onset of freeze-up and ice formation, in autumn, substantial pico-microplankton populations remain in the surface water and are incorporated into the newly formed ice (Eicken 1992; Arrigo and Thomas 2004). Deep vertical mixing and low light intensity suppress water column phytoplankton production during the winter months, during which the biota growing in and on the underside of the sea ice represent an important energy resource for krill larvae and adults (Eicken 1992; Quetin and Ross 2003; Flores et al. 2012a). Unlike adults, larval krill cannot employ survival strategies such as utilizing storage lipids or reducing metabolism or protein catabolism when starving. Therefore, sea ice resources are considered critical for the winter survival of larval and juvenile krill (Daly 1990; Meyer et al. 2002b; Meyer 2012). This dependency can also explain the positive correlation between sea ice extent and population size (Atkinson et al. 2004). The dependency of larval krill on sea ice makes krill an important link between the ice and other environments by feeding on ice organisms, by excreting faeces to the water column and benthos, and by serving as an important food source to predators (Eicken 1992; Van Franeker et al. 1997; Flores et al. 2012a).
Despite the recognized role of sea ice in krill’s life cycle, information on how krill utilize and interact with the sea ice–ocean environment remains limited. There is evidence that the interaction of krill with sea ice varies with sea ice properties (Murphy et al. 2004), season, region, and developmental stage of the krill (Quetin et al. 1994; Murphy et al. 2004; Flores et al. 2012a). These factors could have an effect on the distribution of E. superba (Nicol 2006). Observed distribution patterns of different krill size classes may be attributed to advection from different krill stock sources (Siegel 2012), differences in physiology, e.g. swimming ability and/or transport mechanisms due to different environmental conditions. This will be influenced by the timing of krill spawning. A combination of behavioural and physical factors can cause spatial aggregation of krill of a certain size range or maturity (Kils 1979; Quetin and Ross 1984; Daly and Macaulay 1991), resulting in schools or swarms with similarly sized individuals (Watkins 2000; Kawaguchi et al. 2010).
The onset of krill spawning is influenced by winter sea ice extent and the duration of the sea ice cover (Pakhomov 2000; Siegel 2000). The duration of the spawning season and the number of spawning episodes that occur within one season can be variable (Ross and Quetin 1986; Spiridonov 1995). In general E. superba releases eggs from mid-December to April (Ross and Quetin 1986), with the highest intensity in late December and January (Pakhomov 1995; Spiridonov 1995). The larvae have a complex developmental process going through several stages, namely nauplius I–II, metanauplius, calyptopis I–III and furcilia I–VI (Fraser 1936; Bargmann 1945; Marr 1962; Jia et al. 2014).
Late-stage furcilia (III–VI) have been reported during the onset of winter within the marginal ice zone of the Scotia and Weddell seas. Here, furcilia VI were not commonly found before August, effectively about 150–180 days after the spawning (Daly 1990; Siegel 2000). Nevertheless, in the Bransfield Strait furcilia VI larvae have been found as early as the beginning of winter, and are numerous by spring (Ross and Quetin 1986). During their first winter/early spring furcilia generally develop into juveniles (age class 0) at a length of approximately 15 mm (Siegel 1987). The krill remain in the juvenile stage in their second year (age class 1). At the end of their second year the juveniles become sub-adults, and from the third year onwards, all krill are mature adults (Siegel 1987). Post-larval krill can have a great overlap in size. Juveniles can grow up to 36 mm (Siegel 1987), while females can become mature from 33 mm onwards (Siegel 2012).
Knowledge on the abundance and distribution of different age classes of krill, as well as the interaction of krill with sea ice, is crucial for better predictions of krill recruitment and understanding krill population structure and krill dispersal, particularly in the face of potential sea ice reductions due to climate change (Brierley et al. 2002; Daly 2004; Ross et al. 2004; Sologub and Remelso 2011; Flores et al. 2012b). Pelagic trawls generally undersample the top 1–10 m of the water column, and hydro-acoustic technology is also lacking the ability to explore the upper metres of the water column (Pakhomov 2000; Brierley et al. 2002; Flores et al. 2012a). Therefore, earlier length–frequency analyses of krill, which can be important to find connections between sub-populations, have probably underestimated late larval and early juvenile krill due to a general undersampling of the surface layer and, in particular, the sea ice underside (Melnikov and Spiridonov 1996; Frazer et al. 2002; Atkinson et al. 2008; 2012; Kawaguchi et al. 2010). To overcome this limitation a Surface and Under Ice Trawl (SUIT) was used in this study, enabling large-scale sampling of the upper two metres of the water column under the sea ice (Van Franeker et al. 2009; Flores et al. 2012a; 2014).
The macrozooplankton/micronekton community residing within the under-ice surface layer has previously been shown to differ from the epipelagic layer in terms of species composition, community structure and species density (Flores et al. 2012a, 2014). In this study, krill assemblages were investigated from different depth strata of the northern Weddell Sea during austral winter/early spring. Specifically, we aimed to characterize the population structure of krill at the sea ice interface in terms of length and developmental stage composition and examine habitat partitioning of different krill life stages between the sea ice interface and the water column. Using a comparative approach, we aim to improve our understanding of the relative importance of the sea ice–ocean interface in the life cycle of krill.
Methods
Sample and environmental data collection
Sampling was performed in the northern Weddell Sea during research cruise PS81 (ANT-XXIX/7) on board RV Polarstern, between 24 August and 2 October 2013 (Fig. 1a). The upper two metres of the water column directly under the sea ice were sampled using a Surface and Under Ice Trawl (SUIT, Van Franeker et al. 2009). The trawl has a steel frame with a 2 × 2 m net opening, with a 7-mm half-mesh commercial shrimp net over 1.5 m width, and a 0.3-mm mesh plankton net over 0.5 m width. Floats attached to the top of the frame keep the net at the surface or directly under the ice. The SUIT shears out to the side of the ship, sampling away from the ship’s wake and under relatively undisturbed sea ice (Van Franeker et al. 2009; Suppl. mat. in Flores et al. 2012a).
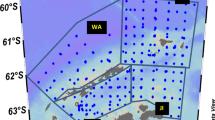
Spatial pattern in Euphausia superba size frequencies. a SUIT and RMT sampling locations, indicated with their station numbers, with ice concentration on 16 September 2013. Dashed rectangles show the spatial size distribution of the krill. ‘Sub’ represents the station dominated by sub-adult krill; 1–3 are the stations dominated by AC0 krill, grouped in stations with similar krill size distributions according to the cluster analysis. b length–frequency distributions of krill as in mapped clusters
The SUIT frame is equipped with a sensor array containing an acoustic Doppler current profiler (ADCP, Nortek Aquadopp®, Norway), which measures the velocity and direction of water passing through the net, and a CTD probe (CTD75 M, Sea & Sun Technology, Germany) with built-in fluorometer (Cyclops, Turner Designs, USA), which measures water temperature, salinity and water column chlorophyll a concentration. Data gaps in the CTD measurements caused by low battery voltage were filled using complementary datasets from the shipboard sensors (temperature, salinity and chlorophyll a at stations 557_2, 560_2 and at station 562_5 only for chlorophyll a), using correction factors determined by linear regression. Connected to the CTD probe was an altimeter (PA500/6-E, Tritech, UK) which measured the distance between the net and the sea ice underside, and it was used to calculate ice thickness. Gaps in the data were filled by constructing a linear model between the CTD ice thickness and ADCP depth in order to derive ice thickness from ADCP depth alone. The set of values for sea ice thickness along a sampling profile was used to calculate a sea ice roughness coefficient. A detailed description of the acquisition and calculation of environmental parameters can be found in David et al. (2015). Regional gridded sea ice concentrations during SUIT hauls were calculated from AMSR2 satellite data, which were acquired from the sea ice portal of the Alfred Wegener Institute (AWI, www.meereisportal.de), using the algorithm from Spreen et al. (2008). The measured and calculated environmental parameters are shown in Table S1 in Online Resource 1.
In total 11 under-ice SUIT stations were completed, four during the day and seven at night. Net tows were conducted at a speed of 1.8–3 km. The volume of water filtered was estimated for each haul by multiplying the mean current velocity with the trawl duration and the opening area, and it ranged between 558.10 and 3177.83 m3 for the plankton net. Further details of sampling are given in Online Resource 1 (Table S1).
The 0- to 500-m stratum was sampled in ice-covered waters with double oblique hauls using a rectangular midwater trawl (RMT). The trawl consisted of an RMT-1 with a 0.33-mm mesh mounted above an RMT-8 with a mesh size of 4.5 mm at the opening and 0.85 mm at the cod end. The net openings were 1 and 8 m2, respectively. A flowmeter (Hydro Bios, Kiel) was mounted in the mouth of the RMT-8 to measure the water volume filtered. Net tows were conducted at a speed of 2–2.5 km. Nine hauls were completed in ice-covered areas, six during the night, two during the day and one during twilight. The volume of water filtered by the RMT-1 ranged between 1055 and 4280 m3. Further details of sampling are given in Online Resource 1 (Table S2).
Samples for krill length–frequency analysis from both nets were preserved in a 4 % hexamine-buffered formaldehyde–sea water solution. Krill from all samples were counted, and total length was measured, to the nearest mm, from the anterior margin of the eye to the tip of the telson (Discovery method; Marr 1962). Larvae were staged based on the number of terminal spines on the telson according to Kirkwood (1982). Krill that have lost one pair of post-lateral spines from their telson (Fraser 1936), but do not show sexual characteristics yet (Makarov and Denys 1981) are defined as juveniles. The sexual maturity of post-larval individuals was further staged according to Makarov and Denys (1981).
Because the SUIT’s shrimp net and the RMT-8 undersample small krill (<20 mm; Siegel 1986; Flores et al. 2012a) and the catch of larger krill was low throughout the sampling area, only data from the SUIT’s plankton and the RMT-1 nets were used for further analyses. As a result, abundances of larger krill (>20 mm) were likely underestimated (Siegel 1986), and this study focuses on larval and juvenile krill that are born in the preceding summer, and which are further referred to as age class 0 (AC0) krill. For comparison between stations and nets, areal and volumetric densities were calculated (ind.m−2 and ind.m−3, respectively).
Statistical analysis
Cluster analysis was performed to analyse similarity of length class frequencies between stations, using Euclidean distance. In order to compare length distributions regardless of varying krill abundances at each station, numbers were standardized to percentages. Stations were grouped using the average linkage method, which was found to work well with suspected unequal cluster sizes and small sample sizes (Ferreira and Hitchcock 2009; Saraçli et al. 2013).
Differences in mean length within stages and between clusters were investigated using one-way ANOVA. Between-group differences were assessed with the Tukey HSD post hoc test. Differences in total abundance per sampling depth were investigated using the nonparametric Wilcoxon rank-sum test due to unequal variances. Differences in size distribution between depth layers were investigated using the Kolmogorov–Smirnov test.
A mixture distribution was fitted to the total catch per size class in ind.1000 m−3, using the maximum likelihood fitting programme CMIX (De la Mare 1994). This model assumes that the sampled population is a mixture of cohorts or age classes and that each group can be described by a parametric distribution. The model provides relative abundance estimates for each cohort (Shelton et al. 2013). The best fitting model was further evaluated using a Chi-square goodness-of-fit test.
All analyses were performed using R statistical software, version 3.0.3 (R Core Team 2014). The CMIX R package was downloaded from the Australian Antarctic Division website (http://www.antarctica.gov.au/science/southern-ocean-ecosystems-environmental-change-and-conservation/southern-ocean-fisheries/fish-and-fisheries/conservation-and-management/cmix).
Results
Environmental conditions
At the western side of the sampling area, the sea ice extended to ~59°S in August and increased to ~58°S from mid-September onward. At the eastern side, the sea ice extent increased to ~56°S in September. Under-ice water properties, measured with the SUIT sensor array, showed low variability throughout the sampling area. Surface temperatures and salinities were on average −1.84 ± 0.012 °C and 34.14 ± 0.11, respectively. Chlorophyll a concentrations of the subsurface waters ranged from 0.097 to 0.134 mg m−3 during August/early-September and showed somewhat higher values ranging from 0.164 to 0.275 mg m−3 during late-September/beginning of October. Sea ice concentrations, in the sampling area, were in general between 86 and 100 %, except at the end of September/beginning of October, when four stations (570–579) where sampled north of 60˚S. At these stations, sea ice coverage decreased to about 50 %. Although the size of the ice floes decreased, sea ice thickness was still within the range (0.30–0.70 m) of the preceding stations, with one exception (station 571_2, 0.23 m). The sea ice roughness coefficient ranged from 0.8 to 3.7, with the highest values at stations 555_47 and 565_5. Snow cover was present at all stations ranging from 0.05 to 0.6 m. Further details of sea ice parameters and snow cover are given in Online Resource 1 (Table S1).
Stage composition and length–frequency distribution of krill in the under-ice surface layer
At most stations sampled with the SUIT, furcilia VI was the dominant stage. Furcilia V and juveniles also had relatively high proportional abundances. Low proportional abundances were recorded for furcilia IV, sub-adult and adult stages. Figure 2 shows the total length and stage composition of krill larvae in the 0- to 2-m depth layer. Sub-adults and adults were only caught in the under-ice surface layer at night, which is consistent with the findings in a previous winter study in the Lazarev Sea (Flores et al. 2012a). Station 551_1 was the only station where mostly sub-adults were caught. The other stations consisted of predominantly AC0 krill. Cluster analysis revealed that these AC0-dominated stations can be divided into three geographically distinct groups, which differed in krill size and developmental stage composition (Figs. 1a, 3). The first cluster consisted of station 555_47, which was dominated by juveniles with a mean length of 15.69 mm (Figs. 1b, 3). The second cluster consisted of stations dominated by furcilia VI (Figs. 1b, 3). Small numbers of juveniles, and occasionally sub-adults, adults and furcilia V were also present in the second cluster stations. The mean length of the krill in this cluster was 11.85 mm. The third cluster was dominated by furcilia V and VI with a mean length of 7.92 mm and had a relatively large proportion of furcilia IV (Figs. 1b, 3). Although there was an overlap in developmental stages among clusters, the average length per developmental stage differed spatially (Fig. 4). The average size of furcilia V and VI was significantly different between each cluster (ANOVA F = 505.9, p < 0.001; Tukey HSD, p < 0.001). Furcilia V were significantly larger in Cluster 2 than in Cluster 3, and absent from Cluster 1. Furcilia VI were significantly larger in Cluster 1 than in Cluster 2 and 3, and smallest in Cluster 3 (Fig. 4). For juveniles, the average size in station 551_1 was significantly larger than all other stations (ANOVA F = 57.11, p < 0.001; Tukey’s HSD, p < 0.02).
Dendrogram of the cluster analysis comparing the similarity of the length distribution of AC0 Euphausia superba in the upper 2 m of the water column under ice. The left cluster consists of a station dominated by juveniles, the middle cluster consists of stations dominated by furcilia VI and the right cluster consists of stations with furcilia IV, V and VI
Size characteristics of the three most abundant stages of Euphauisa. superba (furcilia V, VI and juveniles) per cluster in the SUIT catches. Clusters are defined as in Figs. 1a, 3. The horizontal black lines show the median length in a cluster. The upper and lower limits of the coloured squares indicated the 25th and 75th ‰. The upper and lower limits of the vertical line indicate the minimum and maximum length of that stage in a cluster. Black dots represent the true minimum and maximum lengths, but are numerically distant from the other data points and therefore considered outliers. N represents the number of individuals
Comparison of the under-ice surface to the 0- to 500-m stratum
Krill volumetric density (ind. m−3) was significantly higher in the 0- to 2-m under-ice surface layer than in the 0- to 500-m stratum (Wilcoxon, U = 97, p < 0.001; Fig. 5a), while areal density (ind. m−2) was significantly higher in the 0- to 500-m stratum than in the under-ice layer (Wilcoxon, U = 18, p = 0.016; Fig. 5b). Average abundance estimates from the SUIT catches ranged from 0.09 to 3.60 individuals m−2 and in the RMT catches from 0 to 46.03 individuals m−2. A summary of both depth layers and average length per station is given in Online Resource 1 (Tables S3 & S4). The length–frequency distribution of the part of the population sampled in the 0- to 500-m stratum differed significantly from the distribution in the upper 2 m under the ice (KS test, D(47) = 0.47, p < 0.001). The proportion of small furcilia (<10 mm) in the 0- to 500-m stratum was lower compared to the under-ice layer, while the opposite pattern was observed for larger krill (15–20 mm, Fig. 6).
Comparison of the volumetric density in ind. m−3 (a) and areal density in ind.m−2 (b) of Euphausia superba in the surface layer (0–2 m) and the 0- to 500-m layer under ice. The horizontal black lines show the median density in a depth stratum. The upper and lower limits of the grey squares indicated the 25th and 75th ‰, and thus 50 % of all stations have densities between these limits. The upper and lower limits of the vertical line indicate the minimum and maximum density of the stations in a depth stratum. Black dots represent the true minimum and maximum densities, but are numerically distant from the other data points and therefore considered outliers
Densities at night did not statistically differ from densities during the day (Wilcoxon, 0–2 m: U = 10, p = 1; 0–500 m: U = 3, p = 0.4, Fig S1, Online Resource 2). The total size distribution in the 0- to 2-m depth layer of age class 0 (AC0) krill at night was not significantly different from the distribution at day (KS test, D(20) = 0.25, p = 0.56). Although statistically there was also no difference in the day and night size distribution in the 0- to 500-m depth layer (KS test, D(20) = 0.35, p = 0.17), AC0 krill <8 mm and >15 mm were not found in this depth stratum during the day but only at night (Fg. S2, Online Resource 2).
The cohort mean lengths as determined by the mixture distribution analyses were similar for both SUIT and RMT samples (Fig. 7). The mixture distribution analysis derived from CMIX (De la Mare 1994) showed that the best fit of expected densities vs. observed densities was obtained with four components (0–2 m: χ 2 = 0.997, 0–500 m: χ 2 = 0.999; Fig. 7). One component represented sub-adults and adults, which were 1+ years old. The other components were krill larvae and juveniles that were in their first year, indicating that they represented three separate cohorts. Comparing the cohort mean sizes as determined from the mixture distribution analysis with the clusters using measured krill body length, indicated that there was one cohort (mean length 0–2 m: 7.27 mm, 0–500 m: 7.06 mm) that corresponds with the third cluster containing furcilia IV, V and small furcilia VI. One cohort (mean length 0–2 m: 9.90 mm, 0–500 m: 10.90 mm) corresponds with the second cluster of mainly furcilia VI. The last cohort (mean length 0–2 m: 14.42 mm, 0–500 m: 15.42 mm) corresponds with the first cluster that contains furcilia VI and AC0 juveniles.
Size–frequency distribution of Euphausia superba from different depth layers analysed using CMIX. Grey bars show the observed distribution, and the black line shows the expected distribution (total) which can be subdivided into four components or age groups (dark grey dashed lines). Mean krill lengths and standard deviation (in parentheses) of all components are shown within the figure. a 0- to 2-m layer (χ 2 = 0.997). b 0- to 500-m layer (χ 2 = 0.999)
Discussion
Krill population structure
Primarily AC0 krill were found in both the under-ice surface (0–2 m) and the 0- to 500-m strata of the northern Weddell Sea during winter/early spring. The comparison of krill abundances should be considered with caution because the effect of, e.g., towing speed, sea ice conditions or other factors on the catch efficiency of both nets is not precisely known (Flores et al. 2012a, 2014). Hunt et al. (2014) showed that, at least in the eastern part of the sampling area, AC0 krill were migrating from the ice–water interface down to <20 m at night. Therefore, it should be kept in mind that the AC0 krill found in the 0- to 500-m stratum are most likely still caught in the upper part of the water column (Hunt et al. 2014). Additionally, there is probably a degree in overlap of krill found in both depth layers due to diel vertical migration. It should also be kept in mind that the RMT samples in the wake of the ship and therefore in disturbed sea ice conditions. Earlier comparisons of SUIT and RMT data also indicated that the RMT does not sample the ice–water interface well (Flores et al. 2011, 2012a, 2014). Abundances calculated from the SUIT catch are probably underestimations due to the low efficiency of the SUIT to sample krill from sea ice crevices and over-rafted ice floes, where larval krill have been found to reside (Frazer et al. 2002; Meyer et al. 2009; Flores et al. 2012b). Although krill abundances at stations where the sea ice was relatively rough were not lower than in other stations, the abundance estimates in 0- to 2-m depth stratum presented in this study should be regarded as a minimum estimates.
In the upper 0–2 m furcilia VI were most abundant, while in the 0- to 500-m stratum juveniles dominated. Previous studies, conducted in the Scotia/Weddell Sea and western Antarctic Peninsula (WAP) during late winter/early spring, also found furcilia VI as the dominant stage in the under-ice surface, which was sampled using 1 m2 Ring and Reeve nets and/or divers (Daly 1990; Quetin et al. 2003; Ross et al. 2004). The majority of furcilia VI from our study were similar in size or even larger than those found in other winter studies, and in some instances, our furcilia VI were comparable to studies done in autumn (Daly 2004, Ross et al. 2004 and references therein).
Juveniles in the present study had a size distribution comparable to juveniles sampled from the WAP during January 2002 (Siegel et al. 2003) and were larger than juveniles found in the Scotia/Weddell Sea during winter (Daly 2004), but also than juveniles found in ice–water interface in the western Weddell sea in March/April (Melkinov and Spiridonov 1996). In the latter study, furcilia VI were also abundant, however, in contrast to the juveniles, on average larger than the individuals found in our study (Melkinov and Spiridonov 1996). The larger size range of the furcilia VI and AC0 juveniles of our study compared to the smaller size range reported by Melkinov and Spiridonov (1996) could possibly be explained by the former belonging to different cohorts, while the latter potentially belongs to the same or less cohorts.
Remarkably, our results show that size per developmental stage differed between stations. Furcilia IV, V and VI caught at stations 565_5, 567_2 and 570_5 were small compared to late-stage furcilia caught in other winter studies. Quetin et al. (2003) found that larval krill collected during September of two different years had the same developmental stage despite showing clear differences in total length. They also documented that larvae collected from underneath the ice in July were significantly larger than those collected from open water, although their developmental stages were the same. This suggested that developmental progression may be temporally less variable than the growth rate (Quetin et al. 2003; Pakhomov et al. 2004). Differences in developmental stages and sizes within developmental stages could be explained by dissimilar timing of spawning or different growth conditions of the larval krill caused by variable encountered environmental conditions (Quetin and Ross, 2003). Both could be a result of either multiple spawning episodes arising from a prolonged reproductive season, and therefore multiple cohorts originating from the same population, or the influx of larval krill from different locations (Quetin and Ross 2003). This would also explain the various cohorts we found within the AC0 krill population as established by the mixture distribution and cluster analyses.
The appearance of multiple developmental stages within the clusters indicated that there were at least three cohorts, though it is possible that these cohorts comprised multiple age groups that merged during an earlier stage. Growth experiments have demonstrated that AC0 krill show little or no growth in winter and that inter-moult periods can increase from 19 days in autumn to 40 days in winter, although the latter depends on the feeding conditions (Quetin et al. 2003; Daly 2004). This indicates that length per developmental stage in winter could potentially be more influenced by conditions that allow larvae to achieve a greater length and weight prior to overwintering (Quetin et al. 2003). Therefore, the observed variability of size within larval stages may possibly be a result of differences in environmental conditions experienced by larvae, such as different feeding conditions or unequal growth periods, before the onset of winter.
Drivers of larval distribution
Both the timing of reproduction and the geographic origin of the AC0 krill population influence their transport and therefore their distribution pattern. The large-scale distribution of furcilia has often been assumed to be affected primarily by sea surface circulation (Daly 1990; Pakhomov 2000). However, as young krill are known to reside at the ice–water interface, within crevices and unconsolidated ridges along the under-ice surface or between over-rafted ice floes, the transport and distribution of krill could also be influenced by ice drift (Daly 1990; Thorpe et al. 2007; Meyer et al. 2009). Sea ice moves differently than the underlying water mass, due to the combined influence of wind, ocean currents and mechanical stress (Thorpe et al. 2007). In addition, the ocean surface temperature and salinity gradients are modified by the ice such that the surface currents are very different to those expected when sea ice is not present (Murphy et al. 2004). If larval transport is affected by a combination of surface currents and sea ice drift, then larval transport processes are even more complex than previously assumed, since they depend on sea ice extent, and the locations of boundaries and fronts (Fach et al. 2002).
Particle-tracking models using ocean circulation and sea ice drift patterns (Murphy et al. 2004; Thorpe et al. 2007) suggest that larvae are possibly carried into the northern Weddell Sea area with north-easterly currents from major spawning grounds in the Scotia Sea, north and west of the Antarctic Peninsula, or from the central or south-eastern Weddell Sea, adjacent to the Lazarev Sea (Daly 1990; Melkinov and Spiridonov 1996). Reproduction in the Scotia Sea and the Antarctic Peninsula area typically starts earlier than in the south-eastern Weddell and Lazarev Seas (Spridonov 1995). It is thus a possibility that the larger and older krill from cluster 1 could have originated from the Scotia Sea or the tip of the Antarctic Peninsula, advected by the eastward-flowing current of the northern Weddell Gyre. The smaller and younger krill from cluster 3 may be a result of spawning in the central Weddell Sea. This depends, however, strongly on environmental conditions (Murphy et al. 2004). If the krill larvae remain in the upper layers of the water column, the ocean circulation and ice drift models suggest that larvae originating from the Scotia Sea would potentially move northward. However, in some years it was found that the krill from this flow would be transported back around the South Sandwich Islands into the Weddell Sea (Murphy et al. 2004).
The general pattern of sea ice drift in the northern Weddell Sea would indicate that krill associated with the sea ice would have come from farther south within the Weddell Sea (Murphy et al. 2004; Schwegmann et al. 2011). The reproductive season in the Weddell and Lazarev Seas is typically short due to sea ice conditions. The northward extension of sea ice in the Weddell Sea during January 2013, however, was unusually high and sea ice melt was slow (Vizcarra 2013). This could have caused a spatially less synchronized maturation rate of the krill and thus a higher variation in timing of krill mating, likely leading to an increase in the number of spawning episodes (Spiridonov 1995). It is therefore also a possibility that all the AC0 krill found in our study have originated from within the Weddell Sea and that the different cohorts are a result of a long reproductive season with multiple spawning episodes. More research on the larval origin in the northern Weddell Sea is required.
Comparison of different depth layers
Our results show that the size composition of AC0 krill in in the upper two meters underneath the sea ice was different from the rest of the water column (0–500 m). Although absolute size ranges were similar in both depth layers, the size and stage structure of krill sampled from deeper waters was skewed more towards juvenile krill, while krill sampled from the ice–water interface layer were skewed more towards furcila IV, V and small furcilia VI. A similar pattern was observed west of the Antarctic Peninsula by Frazer et al. (2002), who observed a higher proportion of AC0 juveniles in the 0- to 300-m depth range compared to larval/juvenile krill collected by divers within the under-ice surface layer during late winter.
Differences in sea ice association, overwintering strategies, and/or vertical migration between larval krill and adult krill have been previously noted (Nast 1979; Daly and Macauley 1991; Quetin et al. 1994; Meyer et al. 2002a, 2010; Flores et al. 2012a). Most studies, however, only compared larvae or juveniles with post-larval krill or adults, or made no distinction between furcilia stages, AC0 juveniles and/or AC1 juveniles. Based on length–frequency distributions, Daly and Macaulay (1991) suggested that E. superba in the marginal ice zone make a transition from living in close proximity of the ice–water interface to the epipelagic zone when they reach ~ 25 mm in length. However, no late-stage furcilia were caught in their study, and hence, no comparison could be made between late-stage furcilia and AC0 juveniles (Daly and Macaulay 1991). It is therefore possible that this transition already starts earlier.
Results of our study suggest that while first-year juveniles may still inhabit the ice–water interface, they already are in the process of transiting to deeper layers or/and increasing the amplitude of their vertical migration. The large proportion of small furcilia in the 0- to 2-m depth layer was found at both day and night in similar abundances. Regarding the 0- to 500-m stratum, we note from the outset that there were only 2 daytime RMT tows. Differences in size structure between day and night, and between nets, were therefore in all likelihood influenced by differences in horizontal distribution and the small sample size. Keeping this caveat in mind, it is apparent that the ≤8-mm-size class was completely absent from the day time RMT tows, when the SUIT demonstrated that this population sector was abundant at the sea ice surface (Figure S2). The appearance of the relatively small proportion of ≤8 mm krill and of >15 mm krill in the 0–500 m at night agrees well with the SUIT data (and multi-net data from Hunt et al. 2014) which demonstrated a downward nighttime migration of at least a portion of the AC0 krill population into the water column below the sea ice, where they would be caught by the RMT.
Frazer et al. (2002) proposed that behavioural or physiological differences associated with developmental stages may be responsible for the different larval and juvenile proportions observed in the different depth layers. It has been suggested that the downward movement of euphausiids results from passive sinking and that this behaviour is used to save energy (Rudjakov 1970; Youngbluth 1975). Krill is a relatively heavy species that uses a considerable amount of its energy to maintain at a constant depth. It is also documented that krill density and sinking speed increases with size (Kils 1982). As a consequence their energy expenditure to remain at a fixed depth increases exponentially with body weight. Additionally, the ability of larvae to withstand poor food conditions increases with age (Daly 2004) suggesting that krill, as it matures, would benefit from saving energy by sinking during a passive stage, instead of maintaining its position near the under-ice surface. This would also provide growing krill with access to a larger foraging field which is beneficial in the highly patchy environment.
The vertical distribution of krill appears to be a constant trade-off between food availability, energy budget and predation risk (Youngbluth 1975; Quetin et al. 1996; Watkins 2000; Ross et al. 2014). Sub-adult and adult krill show variation in vertical migration behaviour and depth of occurrence, depending on region and season (Mauchline and Fisher 1969; Marr 1962; Pakhomov 1995; Watkins 2000; Flores et al. 2012a; Siegel 2005). In a multi-seasonal study from the Lazarev Sea comparing the surface layer with deeper depth strata, the post-larval E. superba distribution patterns are variable and different from that of AC0 krill (Flores et al. 2014). The trade-off of AC1 juveniles and (sub-) adults is likely different from that of larvae and AC0 juveniles due to, e.g., different (vertebrate) predators and/or food requirement (Quetin et al. 1996; Siegel 2005; Flores et al. 2012a).
Conclusions
The Antarctic krill population sampled in the northern Weddell Sea during winter/early spring consisted mainly of late-stage furcilia and AC0 juveniles belonging to multiple cohorts. The different cohorts may reflect the influx of krill sub-populations from several regions or of a prolonged reproductive season resulting in multiple spawning episodes within a region, with variation in the growth of individuals due to environmental variability. In a variable environment, an increase in the number of spawning episodes in a single season would theoretically increase reproductive success (Ross and Quetin 2000). Our findings suggest that the northern Weddell Sea could possibly be an area where sub-populations with different temporal or spatial origin converge. To more accurately understand these processes, investigating the ice–water interface on a larger scale is necessary.
This study provides evidence for variations in the vertical distribution and sea ice association between different developmental stages of AC0 krill during winter. The fact that such differences can already be seen within the first year of E. superba’s life suggests that this transition is gradual. This change is likely a result of physiological and behavioural development and ecophysiological trade-offs, causing larger individuals to gradually disperse into deeper layers, under the conditions prevailing during the present study. The preference for different habitats by krill at different developmental stages likely plays an important role in the large-scale spatial distribution of krill, as transport processes between water column and ice vary (Thorpe et al. 2007). The association of younger krill with sea ice also indicates that the effect of sea ice decline on the survival of AC0 krill over winter may vary between krill with different sizes or developmental stages. Differences found in surface waters and deeper layers suggest that, by sampling predominantly deeper layers with conventional pelagic nets, the composition, distribution and abundance of krill populations may not be adequately represented.
References
Arrigo KR, Thomas DN (2004) Large scale importance of sea ice biology in the Southern Ocean. Antarct Sci 16:471–486
Atkinson A, Siegel V, Pakhomov EA, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Atkinson A, Siegel V, Pakhomov EA, Rothery P, Loeb V, Ross RM, Quetin LB, Schmidt K, Fretwell P, Murphy EJ, Tarling GA, Fleming AH (2008) Oceanic circumpolar habitats of Antarctic krill. Mar Ecol Prog Ser 362:1–23
Atkinson A, Nicol S, Kawaguchi S, Pakhomov E, Quetin L, Ross R, Hill S, Reiss C, Siegel V, Tarling G (2012) Fitting Euphausia superba into Southern Ocean food-web models: a review of data sources and their limitations. CCAMLR Sci 19:219–245
Bargmann HE (1945) The development and life-history of adolescent and adult krill, Euphausia superba. Discov Rep XXIII:103–176
Brierley AS, Fernandes PG, Brandon MA, Armstrong F, Millard NW, McPhail SD, Stevenson P, Pebody M, Perrett J, Squires M, Bone DG, Griffiths G (2002) Antarctic krill under sea ice: elevated abundance in a narrow band just south of ice edge. Science 295:1890–1892
Daly K (1990) Overwintering development, growth, and feeding of larval Euphausia superba in the Antarctic marginal ice zone. Limnol Oceanogr 35:1564–1576
Daly K (2004) Overwintering growth and development of larval Euphausia superba: an interannual comparison under varying environmental conditions west of the Antarctic Peninsula. Deep Sea Res Part II 51:2139–2168
Daly K, Macaulay MC (1991) Influence of physical and biological mesoscale dynamics on the seasonal distribution and behaviour of Euphausia superba in the Antarctic marginal ice zone. Mar Ecol Prog Ser 79:37–66
David C, Lange BA, Rabe B, Flores H (2015) Community structure of under-ice fauna in the Eurasian central Arctic Ocean in relation to environmental properties of sea-ice habitats. Mar Ecol Prog Ser 522:15–32
De la Mare WK (1994) Estimating krill recruitment and its variability. CCAMLR Sci 1:55–69
Eicken H (1992) The role of sea ice in structuring Antarctic ecosystems. Polar Biol 12:3–13
Fach BA, Hofmann EE, Murphy EJ (2002) Modeling studies of Antartic krill Euphausia superba survival during transport across the Scotia Sea. Mar Ecol Prog Ser 231:187–203
Ferreira L, Hitchcock DB (2009) A comparison of hierarchical methods for clustering functional data. Commun Stat Simul C 38:1925–1949
Flores H, Van Franeker JA, Cisewski B, Leach H, Van de Putte AP, Meesters EHWG, Bathmann U, Wolff WJ (2011) Macrofauna under sea ice and in the open surface layer of the Lazarev Sea, Southern Ocean. Deep Sea Res Part II 58:1948–1961
Flores H, Atkinson A, Kawaguchi S, Krafft BA, Milinevsky G et al (2012a) Impact of climate change on Antarctic krill. Mar Ecol Prog Ser 458:1–19
Flores H, Van Franeker JA, Siegel V, Haraldsson M, Strass V, Meesters EHWG, Bathmann U, Wolff WJ (2012b) The association of Antarctic krill Euphausia superba with the under-ice habitat. PLoS One 7:e31775. doi:10.1371/journal.pone.0031775
Flores H, Hunt BPV, Kruse S, Pakhomov EA, Siegel V, Van Franeker JA, Strass V, Van de Putte AP, Meesters EHWG, Bathmann U (2014) Seasonal changes in the vertical distribution and community structure of Antarctic macrozooplankton and micronekton. Deep Sea Res Part I 84:127–141
Fraser FC (1936) On the development and distribution of the young stages of krill (Euphausia superba). Discov Rep XIV:3–190
Frazer TK, Quetin LB, Ross RM (2002) Abundance, sizes and developmental stages of larval krill, Euphausia superba, during winter in ice-covered sea west of the Antarctic Peninsula. J Plankton Res 24:1067–1077
Hunt B, Pakhomov E, Teschke M, King R, Cantzler H, Halbach L, Bose A, Krieger M (2014) Multi-net 24 h stations. In: Meyer B, Auerswald L (eds). The expedition of the research vessel “Polarstern” to the Antarctic in 2013 (ANT-XXIX/7). Reports on Polar and Marine Research 674:14–18
Jia Z, Virtue P, Swadling KM, Kawaguchi S (2014) A photographic documentation of the development of Antarctic krill (Euphausia superba) from egg to early juvenile. Polar Biol 37:165–179
Kawaguchi S, King R, Meijers R, Osborn JE, Swadling KM, Ritz DA, Nicol S (2010) An experimental aquarium for observing the schooling behaviour of Antarctic krill (Euphausia superba). Deep Sea Res Part II 57:683–692
Kils U (1979) Swimming speed and escape capacity of Antarctic krill Euphausia superba. Meeresforschung 27:264–266
Kils U (1982) Swimming behaviour, swimming performance and energy balance of Antartic krill, Euphausia superba. BIOMASS Sci Ser 3:1–121
Kirkwood JM (1982) ANARE Research Notes 1. In: A guide to the Euphausiacea of the Southern Ocean, Australian Antarctic Division, Kingston, pp 45
Makarov RR, Denys CJ (1981) Stages of sexual maturity of Euphausia superba Dana. BIOMASS Handb 11:2–13
Marr J (1962) The natural history and geography of the Antarctic krill (Euphausia superba Dana). Discov Rep XXXII:37–123
Mauchline J, Fisher LR (1969) The biology of euphausiids. Adv Mar Biol 7:1–454
Melkinov IA, Spiridonov VA (1996) Antarctic krill under perennial sea ice in the western Weddell Sea. Antarct Sci 8:323–329
Meyer B (2012) The overwintering of Antarctic krill, Euphausia superba, from an ecophysiological perspective. Polar Biol 35:15–37
Meyer B, Atkinson A, Stübing D, Oettl B, Hagen W, Bathmann UV (2002a) Feeding and energy budgets of Antarctic krill Euphausia superba at the onset of winter-I. Furcilia III larvae. Limnol Oceanogr 47:943–952
Meyer B, Saborowski R, Atkinson A, Buchholz F, Bathmann U (2002b) Seasonal differences in citrate synthase and digestive enzyme activity in larval and postlarval Antarctic krill, Euphausia superba. Mar Biol 141:855–862
Meyer B, Fuentes V, Guerra C, Schmidt K, Atkinson A, Spahic S, Cisewski B, Freier U, Olariaga A, Bathmann U (2009) Physiology, growth, and development of larval krill Euphausia superba in autumn and winter in the Lazarev Sea, Antarctica. Limnol Oceanogr 54:1595–1614
Meyer B, Auerswald L, Siegel V, Spahić S, Pape C, Fach BA, Teschke M, Lopata AL, Fuentes V (2010) Seasonal variation in body composition, metabolic activity, feeding and growth of adult krill Euphausia superba in the Lazarev Sea. Mar Ecol Prog Ser 398:1–18
Murphy EJ, Thorpe SE, Watkins JL, Hewitt R (2004) Modelling the krill transport pathways in the Scotia Sea: spatial and environmental connections generating the seasonal distribution of krill. Deep Sea Res Part II 51:1435–1456
Nast F (1979) The vertical distribution of larval and adult krill (Euphausia superba Dana) on a time station south of Elephant Island, South Shetlands. Meeresforschung 27:103–118
Nicol S (2006) Krill, currents, and sea ice: Euphausia superba and its changing environment. Bioscience 56:111–120
Pakhomov EA (1995) Demographic studies of Antarctic krill Euphausia superba in the cooperation and Cosmonaut Seas (Indian sector of the Southern Ocean). Mar Ecol Prog Ser 119:45–61
Pakhomov EA (2000) Demography and life cycle of Antarctic krill, Euphausia superba, in the Indian sector of the Southern Ocean: long-term comparison between coastal and open-ocean regions. Can J Fish Aquat Sci 57(Suppl. 3):68–90
Pakhomov EA, Atkinson A, Meyer B, Oettl B, Bathmann U (2004) Daily rations and growth of larval krill Euphausia superba in the Eastern Bellingshausen Sea during austral autumn. Deep Sea Res Part II 51:2185–2198
Quetin LB, Ross RM (1984) School composition of the Antarctic krill Euphausia superba in the waters west of the Antarctic peninsula in the austral summer of 1982. J Crustacean Biol Vol. 4:Special No 1. The biology of the Antarctic krill Euphausia superba: proceedings of the First International Symposium on Krill held at Wilmington, North Carolina, from 16 to 19 October 1982 (November 1984), pp 96–106
Quetin LB, Ross RM (2003) Episodic recruitment in Antarctic krill Euphausia superba in the Palmer LTER study region. Mar Ecol Prog Ser 259:185–200
Quetin LB, Ross RM, Clarke A (1994) Krill energetics: seasonal and environmental aspect of the physiology of Euphausia superba. In: Sayed E (ed) Southern ocean ecology: the BIOMASS perspective. Cambridge University Press, Cambridge, pp 165–184
Quetin LB, Ross RM, Frazer TK, Haberman KL (1996) Factors affecting distribution and abundance of zooplankton, with an emphasis on Antarctic krill, Euphausia superba. Antarct Res Ser 70:357–371
Quetin LB, Ross RM, Frazer TK, Amsler MO, Wyatt-Evens C, Oakes SA (2003) Growth of larval krill, Euphausia superba, in fall and winter west of the Antarctic Peninsula. Mar Biol 143:833–843
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ross RM, Quetin LB (1986) How productive are Antarctic krill? Bioscience 36:264–269
Ross RM, Quetin LB (2000) Reproduction in Euphausiacea. In: Everson I (ed) Krill: biology, ecology and fisheries. Blackwell Science Ltd, Oxford, pp 150–181
Ross RM, Quetin LB, Newberger T, Oakes SA (2004) Growth and behavior of larval krill (Euphausia superba) under the ice in late winter 2001 west of the Antarctic Peninsula. Deep Sea Res Part II 51:2169–2184
Ross RM, Quetin LB, Newberger T, Shaw CT, Jones JL, Oakes SA, Moore KJ (2014) Trends, cycles, interannual variability for three pelagic species west of the Antarctic Peninsula 1993–2008. Mar Ecol Prog Ser 515:11–32
Rudjakov JA (1970) The possible causes of diel vertical migrations of planktonic animals. Mar Biol 6:98–105
Saraçli S, Doğan N, Doğan I (2013) Comparison of hierarchical cluster analysis methods by cophenetic correlation. J Inequal Appl 203:1–8
Schwegmann S, Haas C, Fowler C, Gerdes R (2011) A comparison of satellite-derived sea-ice motion with drifting buoy data in the Weddell Sea. Ann Glaciol 52:1–8
Shelton AO, Kinzey D, Reiss C, Munch S, Watters G, Mangel M (2013) Among-year variation in growth of Antarctic krill Euphausia superba based on length-frequency data. Mar Ecol Prog Ser 481:53–67
Siegel V (1986) Untersuchungen zur biologie des Antarkischen krill, Euphausia superba, in bereich der Bransfield Straβe und angrenzender Gebiete. Mitt Inst Seefisch 38:1–244
Siegel V (1987) Age and growth of Antarctic Euphausiacea (Crustacea) under natural conditions. Mar Biol 96:483–495
Siegel V (2000) Krill (Euphausiacea) life history and aspects of population dynamics. Can J Fish Aquat Sci 57(Suppl. 3):130–150
Siegel V (2005) Distribution and population dynamics of Euphausia superba: summary of recent findings. Polar Biol 29:1–22
Siegel V (2012) Krill stocks in high latitudes of the Antarctic Lazarev Sea: seasonal and interannual variation in distribution, abundance and demography. Polar Biol 35:1151–1177
Siegel V, Ross RM, Quetin LB (2003) Krill (Euphausia superba) recruitment indices from the western Antarctic Peninsula: are they representative of larger regions? Polar Biol 26:672–679
Sologub DO, Remelso AV (2011) Distribution and size-age composition of Antarctic krill (Euphausia superba) in the South Orkney Islands region. CCAMLR Sci 18:123–134
Spiridonov VA (1995) Spatial and temporal variability in reproductive timing of Antarctic krill (Euphausia superba Dana). Polar Biol 15:161–174
Spreen G, Kaleschke L, Heygster G (2008) Sea ice remote sensing using amsr-e 89-ghz channels. J Geophys Res Oceans 113:C02S03
Thorpe SE, Murphy EJ, Watkins JL (2007) Circumpolar connections between Antarctic krill (Euphausia superba Dana) populations: investigating the roles of ocean and sea ice transport. Deep Sea Res 54:792–810
Van Franeker JA, Bathmann UV, Matmot S (1997) Carbon fluxes to Antarctic top predators. Deep Sea Res Part II 44:435–455
Van Franeker JA, Flores H, Van Dorssen M (2009) The surface and under ice trawl (SUIT). In: Flores H (2009) Frozen desert alive. Dissertation, University of Groningen, pp 181–188
Vizcarra N (2013) A wintry mix from a dynamic cryosphere. Arctic sea ice news and analysis. National Snow and Ice Data Center, Boulder, Colorado, USA. http://nsidc.org/arcticseaicenews/2013/02/a-wintry-mix/. Accessed 20 Apr 2015
Watkins J (2000) Aggregation and vertical migration. In: Everson I (ed) Krill: biology, ecology and fisheries. Blackwell Science Ltd, Oxford, pp 80–102
Youngbluth MJ (1975) The vertical distribution and diel migration of euphausiids in the central waters of the eastern South Pacific. Deep Sea Res 22:519–536
Acknowledgments
We are very grateful for the support of Captain Stefan Schwarze, officers and crew of Polarstern during expedition ANT-XXIX/7. Special thanks go to Michiel van Dorssen (M. van Dorssen Metaalbewerking) for operational and technical support with SUIT, Martina Vortkamp (AWI) and André Meijboom (IMARES) for technical assistance, Santiago Alvarez-Fernandez (IMARES) for help with statistics, Troy Robertson (Australian Antarctic Division) for help using the CMIX software, Christine Klaas (AWI) for help calibrating chlorophyll a data, and Pascalle Jacobs (IMARES) and three anonymous reviewers for commenting on a previous version of the manuscript. This study was funded by the Netherlands Ministry of EZ (project WOT-04-009-036) and the Netherlands Polar Program (project ALW 866.13.009). The study is associated with the Helmholtz Association Young Investigators Group Iceflux: Ice-ecosystem carbon flux in polar oceans (VH-NG-800) and contributes to the Helmholtz research Programme PACES II, Topic 1.5. Expedition grant no: AWI-PS81_01 (WISKY).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schaafsma, F.L., David, C., Pakhomov, E.A. et al. Size and stage composition of age class 0 Antarctic krill (Euphausia superba) in the ice–water interface layer during winter/early spring. Polar Biol 39, 1515–1526 (2016). https://doi.org/10.1007/s00300-015-1877-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1877-7