Abstract
Key message
Priming alleviates membrane damage, chlorophyll degradation along with cryoprotectants accumulation during chilling stress that leads to improved reproductive functioning and increased seed yield.
Abstract
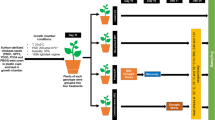
Chilling temperatures below 15 °C have severe implications on the reproductive growth and development of chickpea. The abnormal reproductive development and subsequent reproductive failure lead to substantial yield loss. We exposed five chickpea cultivars (PBG1, GPF2, PDG3, PDG4, and PBG5) to drought stress (Priming) during the vegetative stage and analyzed for chilling tolerance during the reproductive stage. These varieties were raised in the fields in two sets: one set of plants were subjected to drought stress at the vegetative stage for 30 days (priming) and the second set of plants were irrigated regularly (non-primed). The leaf samples were harvested at the flowering, podding, and seed filling stage and analyzed for membrane damage, water status, chlorophyll content, cellular respiration, and certain cryoprotective solutes. The reproductive development was analyzed by accessing pollen viability, in vivo and in vitro germination, pollen load, and in vivo pollen tube growth. Principal component analysis (PCA) revealed that priming improved membrane damage, chlorophyll b degradation, and accumulation of cryoprotectants in GPF2, PDG3, and PBG5 at the flowering stage (< 15 °C). Pearson's correlation analysis showed a negative correlation with the accumulation of proline and carbohydrates with flower, pod, and seed abortion. Only, PBG5 responded best to priming while PBG1 was worst. In PBG5, priming resulted in reduced membrane damage and lipid peroxidation, improved water content, reduced chlorophyll degradation, and enhanced cryoprotective solutes accumulation, which led to increased reproductive functioning and finally improved seed yield and harvest index. Lastly, the priming response is variable and cultivar-specific but overall improve plant tolerance.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Abid M, Tian Z, Ata-Ul-Karim ST, Liu Y, Cui Y, Zahoor R, Jiang D, Dai T (2016) Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and sensitive wheat cultivars. Plant Physiol Biochem 106:218–227
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beck EH, Fettig S, Knake C, Hartig K, Bhattarai T (2007) Specific and unspecific responses of plants to cold and drought stress. J Biosci 32:501–510
Berger J, Kumar S, Nayyar H, Street K, Sandhu JS, Henzell J, Kaur J, Clarke H (2012) Temperature-stratified screening of chickpea (Cicer arietinum L.) genetic resource collections reveals very limited reproductive chilling tolerance compared to its annual wild relatives. Field Crops Res 126:119–129
Bloom A, Zwieniecki M, Passioura J, Randall L, Holbrook N, StClair D (2004) Water relations under root chilling in a sensitive and tolerant tomato species. Plant Cell Environ 27:971–979
Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50:859–865
Bruce TJ, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Gill SS, Gill R, Anjum N (2014) Target osmoprotectants for abiotic stress tolerance in crop plants—glycine betaine and proline. In: Anjum N, Gill SS, Gill R (eds) Plant adaptation to environmental change Significance of amino acids and their derivatives. Cab international, Wallingford
Guo Z, Ou W, Lu S, Zhong Q (2006) Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol Biochem 44:828–836
Hajihashemi S, Noedoost F, Geuns J, Djalovic I, Siddique KH (2018) Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front Plant Sci 9:1430
Hasanuzzaman M, Nahar K, Fujita M (2013) Extreme temperature responses, oxidative stress and antioxidant defense in plants. In: Vahdati K, Leslie C (eds) Abiotic stress–plant responses and applications in agriculture. InTech, Croatia
Havaux M, Kloppstech K (2001) The protective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta 213:953–966
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoffman L, DaCosta M, Ebdon JS, Zhao J (2012) Effects of drought preconditioning on freezing tolerance of perennial ryegrass. Environ Exp Bot 79:11–20
Jan N, Majeed U, Andrabi KI, John R (2018) Cold stress modulates osmolytes and antioxidant system in Calendula officinalis. Acta Physiol Plant 40:73
Kalisz A, Jezdinský A, Pokluda R, Sękara A, Grabowska A, Gil J (2016) Impacts of chilling on photosynthesis and chlorophyll pigment content in juvenile basil cultivars. Hortic Environ Biotechnol 57:330–339
Kaur G, Kumar S, Nayyar H, Upadhyaya H (2008) Cold stress injury during the pod-filling phase in chickpea (Cicer arietinum L.): Effects on quantitative and qualitative components of seeds. J Agron Crop Sci 194:457–464
Kaur S, Jairath A, Singh I, Nayyar H, Kumar S (2016) Alternate mild drought stress (− 0.1 MPa PEG) MPa PEG immunizes sensitive chickpea cultivar against lethal chilling by accentuating the defense mechanisms. Acta Physiol Plant 38:189
Kiran A, Kumar S, Nayyar H, Sharma KD (2019) Low temperature-induced aberrations in male and female reproductive organ development cause flower abortion in chickpea. Plant Cell Environ 42:2075–2089
Knight H, Knight MR (2001) Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci 6:262–267
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608
Kumar S, Nayyar H, Bhanwara R, Upadhyaya H (2010) Chilling stress effects on reproductive biology of chickpea. J SAT Agric Res 8:1–14
Kumar S, Malik J, Thakur P, Kaistha S, Sharma KD, Upadhyaya H, Berger J, Nayyar H (2011) Growth and metabolic responses of contrasting chickpea (Cicer arietinum L.) genotypes to chilling stress at reproductive phase. Acta Physiol Plant 33:779–787
Lewandowska M, Jarvis P (1977) Changes in chlorophyll and carotenoid content specific leaf area and dry weight fraction in Sitka spruce in response to shading and season. New Phytol 79:247–256
Li X, Topbjerg HB, Jiang D, Liu F (2015) Drought priming at vegetative stage improves the antioxidant capacity and photosynthesis performance of wheat exposed to a short-term low temperature stress at jointing stage. Plant Soil 393:307–318
Lutts S, Kinet J, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Martinez-Medina A, Flors V, Heil M, Mauch-Mani B, Pieterse CM, Pozo MJ, Ton J, van Dam NM, Conrath U (2016) Recognizing plant defense priming. Trends Plant Sci 21:818–822
Nathalie V, Christian H (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Nayyar H, Bains T, Kumar S (2005a) Chilling stressed chickpea seedlings: effect of cold acclimation calcium and abscisic acid on cryoprotective solutes and oxidative damage. Environ Exp Bot 54:275–285
Nayyar H, Bains T, Kumar S (2005b) Low temperature induced floral abortion in chickpea: relationship to abscisic acid and cryoprotectants in reproductive organs. Environ Exp Bot 53:39–47
Patton AJ, Cunningham SM, Volenec JJ, Reicher ZJ (2007) Differences in freeze tolerance of zoysiagrasses: II. Carbohydr Proline Accumul Crop Sci 47:2170–2181
Pirzadah TB, Malik B, Rehman RU, Hakeem KR, Qureshi MI (2014) Signaling in Response to Cold Stress. In: Hakeem KR, Rehman RU, Tahir I (eds) Plant signaling: Understanding the molecular crosstalk. Springer, Berlin, pp 193–226
Rajashekar CB, Panda M (2014) Water stress is a component of cold acclimation process essential for inducing full freezing tolerance in strawberry. Sci Hortic 174:54–59
Rejeb IB, Pastor V, Mauch-Mani B (2014) Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants 3:458–475
Rivero RM, Ruiz JM, Garcıa PC, Lopez-Lefebre LR, Sánchez E, Romero L (2001) Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci 160:315–321
Saini R, Adhikary A, Nayyar H, Kumar S (2019) Cross-priming accentuates key biochemical and molecular indicators of defense and improves cold tolerance in chickpea (Cicer arietinum L.). Acta Physiol Plant 41:181
Srinivasan A, Saxena N, Johansen C (1999) Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.): genetic variation in gamete development and function. Field Crops Res 60:209–222
Sulusoglu M, Cavusoglu A (2014) In vitro pollen viability and pollen germination in cherry laurel (Prunus laurocerasus L.). Sci World J 2014:1–7
Taïbi K, Del Campo AD, Vilagrosa A, Bellés JM, López-Gresa MP, López-Nicolás JM, Mulet JM (2018) Distinctive physiological and molecular responses to cold stress among cold-tolerant and cold-sensitive Pinus halepensis seed sources. BMC Plant Biol 18:236
Tewari AK, Tripathy BC (1998) Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol 117:851–858
Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H (2010) Cold stress effects on reproductive development in grain crops: an overview. Environ Exp Bot 67:429–443
Thakur A, Sharma KD, Siddique KH, Nayyar H (2020) Cold priming the chickpea seeds imparts reproductive cold tolerance by reprogramming the turnover of carbohydrates, osmo-protectants and redox components in leaves. Sci Hortic 261:108929
Theocharis A, Clément C, Barka EA (2012) Physiological and molecular changes in plants grown at low temperatures. Planta 235:1091–1105
Turk H, Erdal S, Genisel M, Atici O, Demir Y, Yanmis D (2014) The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul 74:139–152
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Wang X, Vignjevic M, Liu F, Jacobsen S, Jiang D, Wollenweber B (2015) Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul 75:677–687
Wojdyło A, Oszmiański J, Czemerys R (2007) Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 105:940–949
Yamasaki S, Dillenburg LR (1999) Measurements of leaf relative water content in Araucaria angustifolia. Rev Bras De Fisiol Veg 11:69–75
Yang J, Kong Q, Xiang C (2009) Effects of low night temperature on pigments, chl a fluorescence and energy allocation in two bitter gourd (Momordica charantia L.) genotypes. Acta Physiol Plant 31:285–293
Acknowledgements
The authors are thankful to the Central University of Punjab for providing the necessary infrastructure and Punjab Agriculture University for providing chickpea seeds. Authors are thankful to the University Grant Commission (UGC) New Delhi for financial assistance, and Indian Council of Medical Research (ICMR), New Delhi for financial assistance in the form of Junior Research Fellowship.
Funding
This study was funded by University Grant Commission (UGC), New Delhi, Central university of Punjab, Bathinda and Indian Council of Medical Research (ICMR), New Delhi.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. RS has performed the experimental work, data analysis and written the manuscript. RD repeated the field experiment and observations, AA, RK have assisted in field trials, sampling, and biochemical analysis. IS provided authentic germplasm. SK has conceptualized, planned and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Prakash Lakshmanan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saini, R., Das, R., Adhikary, A. et al. Drought priming induces chilling tolerance and improves reproductive functioning in chickpea (Cicer arietinum L.). Plant Cell Rep 41, 2005–2022 (2022). https://doi.org/10.1007/s00299-022-02905-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-022-02905-7