Abstract
Objective
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with a variable clinical manifestation, potentially leading to death. Importantly, patients with SLE have an increased risk of neoplastic disorders. Thus, this study aimed to comprehensively evaluate the clinical and laboratory characteristics of patients with SLE and with or without malignancy.
Methods
We conducted a retrospective analysis of medical records of 932 adult Caucasian patients with SLE treated at the University Hospital in Kraków, Poland, from 2012 to 2022. We collected demographic, clinical, and laboratory characteristics, but also treatment modalities with disease outcomes.
Results
Among 932 patients with SLE, malignancy was documented in 92 (9.87%), with 7 (7.61%) patients experiencing more than one such complication. Non-hematologic malignancies were more prevalent (n = 77, 83.7%) than hematologic malignancies (n = 15, 16.3%). Patients with SLE and malignancy had a higher mean age of SLE onset and a longer mean disease duration than patients without malignancy (p < 0.001 and p = 0.027, respectively). The former group also presented more frequently with weight loss (odds ratio [OR] = 2.62, 95% confidence interval [CI] 1.61–4.23, p < 0.001), fatigue/weakness (OR = 2.10, 95% CI 1.22–3.77, p = 0.005), and fever (OR = 1.68, 95% CI 1.06–2.69, p = 0.024). In the malignancy-associated group, we noticed a higher prevalence of some clinical manifestations, such as pulmonary hypertension (OR = 3.47, 95% CI 1.30–8.42, p = 0.007), lung involvement (OR = 2.64, 95% CI 1.35–4.92, p = 0.003) with pleural effusion (OR = 2.39, 95% CI 1.43–3.94, p < 0.001), and anemia (OR = 2.24, 95% CI 1.29–4.38, p = 0.006). Moreover, the patients with SLE and malignancy more frequently had internal comorbidities, including peripheral arterial obliterans disease (OR = 3.89, 95% CI 1.86–7.75, p < 0.001), myocardial infarction (OR = 3.08, 95% CI 1.41–6.30, p = 0.003), heart failure (OR = 2.94, 95% CI 1.30–6.17, p = 0.005), diabetes mellitus (OR = 2.15, 95% CI 1.14–3.91, p = 0.011), hypothyroidism (OR = 2.08, 95% CI 1.29–3.34, p = 0.002), arterial hypertension (OR = 1.97, 95% CI 1.23–3.23, p = 0.003), and hypercholesterolemia (OR = 1.87, 95% CI 1.18-3.00, p = 0.006). Patients with SLE and malignancy were treated more often with aggressive immunosuppressive therapies, including cyclophosphamide (OR = 2.07, 95% CI 1.30–3.28, p = 0.002), however median cumulative cyclophosphamide dose in malignancy-associated SLE subgroup was 0 g (0–2 g). Interestingly, over a median follow-up period of 14 years (ranges: 8–22 years) a total of 47 patients with SLE died, with 16 cases (5.28%) in the malignancy-associated SLE group and 31 cases (5.73%) in the non-malignancy SLE group (p = 0.76). The most common causes of death were infections (21.28%) and SLE exacerbation (8.51%).
Conclusion
The study highlights the relatively frequent presence of malignancies in patients with SLE, a phenomenon that demands oncological vigilance, especially in patients with a severe clinical course and comorbidities, to improve long-term outcomes in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease, typified by various clinical manifestations that involve the skin, cardiovascular, mucosal, gastrointestinal, hematological, neurological, musculoskeletal, pulmonary, and renal systems [1]. Most patients with SLE present a relapsing-remitting course with a higher risk of developing life-threatening complications; these patients require high-intensity immunosuppressive therapy [2]. In general, patients with SLE have an increased risk of all hematologic cancers, such as lymphoma, leukemia, and multiple myeloma, but also other types of solid malignancy, including lung cancer, liver and hepatobiliary cancer, vaginal and vulvar cancer, thyroid cancer, head and neck cancer, renal cancer, and nonmelanoma skin cancer [3, 4]. Interestingly, one of the most common malignancies in patients with SLE is non-Hodgkin lymphoma, with a 3-4-fold increased risk compared with the general population with an aggressive histologic subtype, such as diffuse large B-cell lymphoma [5, 6]. In turn, patients with SLE have a lower risk of breast, ovarian, and endometrial cancer, a phenomenon that might be related to hormonal factors and specific lupus antibodies [7]. For example, anti-DNA antibodies inhibit DNA repair, which might be toxic to cancer cells with DNA repair defects, making the cells more sensitive to DNA-damaging agents such as doxorubicin and radiation [8]. According to the recent meta-analysis by Zhang et al. [9], cancers occur in patients with SLE 62% more often than in the general population. In a recent study on malignancies in SLE by Kariniemi et al. [10], patients with SLE and malignancy had a lower adjusted 15-year survival than controls with malignancy, 27.1% versus 52.4%.
Investigations into the relationship between cancer risk and specific SLE disease characteristics, such as organ involvement, disease activity, and treatment regimens, are essential for a comprehensive understanding of this complex interplay. Clinical and laboratory biomarkers might serve as valuable indicators of cancer risk. Therefore, we aimed to analyze differences in disease characteristics and laboratory findings between patients with SLE and with or without cancer to search for potential predictors and risk profiles in patients with SLE and malignancy. Exploring the similarities and differences in cancer risk profiles between individuals with SLE and the general population can provide insights into the unique factors contributing to oncogenesis in the context of autoimmune diseases.
Patients and methods
Study population
We retrospectively reviewed medical records of all SLE cases fulfilling the European League Against Rheumatism and American College of Rheumatology (EULAR/ACR) criteria from 2019 [11], who were treated in the University Hospital, Kraków, Poland, from January 2012 to June 2022. Detailed demographic, clinical, and laboratory characteristics of the enrolled patients with SLE are included in our previous publication [12]. In brief, we collected data on sex, current age, age at the first SLE symptoms and disease onset, time delay between the onset of SLE symptoms and diagnosis, duration of the disease, family history of autoimmune diseases, clinical and laboratory SLE manifestations, cause and age of death (if applicable), internal comorbidities, miscarriages in women, and different immunosuppressive treatment modalities. Next, we divided all patients into two subgroups: The first group comprised patients with SLE and a malignancy diagnosis (the malignancy-associated SLE group), whereas the second group consisted of patients with SLE but without a malignancy diagnosis (the non-malignancy-associated SLE group). The evaluated clinical manifestations included: general symptoms, lymphadenopathy, skin lesions, oral or nasopharyngeal ulcerations, photosensitivity, joint involvement, serositis, the hematologic domain (leukopenia, lymphopenia, anemia, hemolytic anemia, thrombocytopenia, macrophage activation syndrome [MAS], and thrombotic thrombocytopenic purpura), lupus nephritis (LN), nervous system involvement, Raynaud’s phenomenon, respiratory system involvement, and lupoid hepatitis; they are described in detail in our previous publication [12]. We extended the evaluation of malignancy in patients with SLE to age at the malignancy diagnosis, the duration between malignancy and the SLE diagnosis, and the type of malignancy. We also analyzed medical comorbidities, such as arterial hypertension, diabetes mellitus, hypercholesterolemia, atrial fibrillation, lower extremity peripheral arterial obliterans disease, heart failure, and any thromboembolic events. The treatment modalities included corticosteroids, hydroxychloroquine or chloroquine, azathioprine, methotrexate, cyclosporine, mycophenolate mofetil, cyclophosphamide, sulfasalazine, immunoglobulins intravenously in suppressive doses, and biological agents (belimumab, rituximab, and anifrolumab), currently or in the past. We also checked whether patients had splenectomy or plasmapheresis.
The Bioethics Committee of the Jagiellonian University Medical College approved the research (No: N41/DBS/000936). All procedures adhered to the ethical principles outlined in the Declaration of Helsinki.
Laboratory analysis
A complete blood cell count, creatinine with estimated glomerular filtration rate (eGFR) determined by the Modification of Diet in Renal Disease formula, 24-hour urine protein excretion, urinary sediment analysis, lipid profile, haptoglobin, direct antiglobulin test, and blood group designation were analyzed using standard laboratory techniques [13]. Anti-nuclear antibodies (ANA) were assessed through indirect immunofluorescence (IIF). Specific antibodies, including anti-SSA, anti-SSB, anti-histone, anti-nucleosome, anti-Smith (Sm), and anti-ribonucleoprotein (RNP), were identified by using enzyme-linked immunosorbent assays (ELISAs) or line-blot immunoassays. Anti-double-stranded DNA (anti-dsDNA) antibodies were assayed via IIF with Crithidia luciliae as the substrate. The anti-myeloperoxidase (MPO) and anti-proteinase 3 (PR3) antibodies were evaluated with a standardized ELISA technique. Serum complement levels (C3c and C4) and rheumatoid factor (RF) were determined using nephelometry. In a retrospective analysis, laboratory tests for hypercoagulability were conducted, encompassing assessments for lupus anticoagulant (LA), anti-cardiolipin (aCL), and anti-beta-2-glycoprotein I (aβ2GPI) antibodies in both the IgM and IgG classes. In addition, antithrombin activity, protein C activity, free protein S level, and factor VIII activity were evaluated, and factor V Leiden and prothrombin G20210A gene variants were identified. All measurements were performed using standard routine laboratory techniques.
Statistical analysis
We analyzed the results by using STATISTICA Tibco 13.3 and the R software. Categorical variables are presented as frequencies (number of cases) with relative frequencies (percentages), and were compared using the Chi2 test or Fisher’s exact test. The normality of the data distribution was evaluated using the Shapiro-Wilk test. All continuous variables were non-normally distributed; thus, they are presented as the median with Q1-Q3 ranges and were compared using the Mann-Whitney test. We calculated the odds ratio (OR) with 95% confidence interval (CI) by determining the cut-off points based on receiver operating characteristic (ROC) curves. We considered a two-sided p < 0.05 to be statistically significant for all analyses.
Results
Demographic features
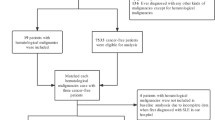
The study included 92 patients with SLE and malignancy, constituting 9.87% of the total SLE cases (n = 932). Additionally, 7 (7.61%) of the patients with SLE and malignancy had at least two malignancies in their medical records.
Table 1 presents the cumulative demographic characteristics of the SLE cohort. The sex distribution between the malignancy-associated and non-malignancy-associated SLE groups was not significantly different (p = 0.72). Patients with confirmed malignancy were diagnosed with SLE a median of 13 years earlier than those without malignancy (46 and 33 years, respectively, p < 0.001), but the disease duration was comparable between the analyzed groups (14 and 12 years, respectively, p = 0.40). The time delay between symptom onset and diagnosis was similar in both SLE groups (median of 0 months for both, p = 0.09), while the first symptoms in the malignancy-associated group appeared at a median of 12 years earlier than in the non-malignancy-associated group (42 and 30 years, respectively, p < 0.001).
Among the patients with SLE, 157 (17.05%) had a positive family history of any autoimmune diseases. In the malignancy-associated and non-malignancy-associated SLE groups, rheumatoid arthritis, SLE, psoriasis, and Hashimoto’s disease were the most prevalent, with no significant differences between the groups (p > 0.05 for all). Other autoimmune diseases included systemic sclerosis, granulomatosis with polyangiitis, mixed connective tissue disease, Sjögren’s syndrome, ulcerative colitis, celiac disease, Graves’ disease, myasthenia gravis, immune thrombocytopenia, autoimmune hepatitis, dermatomyositis, Addison-Biermer anemia, and type I diabetes mellitus; they also showed no significant differences between the groups (p > 0.05 for all).
A more severe course of SLE in patients with an accompanying malignancy
Table 2 provides an overview of the cumulative frequencies of systemic involvement in the SLE cohort. In the malignancy-associated SLE group, the most prevalent clinical manifestations included hematological symptoms (93.42%), joint issues (90.22%), and general symptoms (89.13%). The non-malignancy-associated SLE group exhibited comparable predominant symptoms, with hematological symptoms (91.2%), joint problems (89.89%), and mucocutaneous manifestations (83.12%) being the most frequent. A comparison between the malignancy-associated and non-malignancy-associated SLE groups revealed that the former often presented with more severe clinical manifestations. Specifically, patients with SLE and malignancy exhibited a higher incidence of weight loss (OR = 2.62, 95% CI 1.61–4.23, p < 0.001), pleural effusion (OR = 2.39, 95% CI 1.43–3.94, p < 0.001), fever (OR = 1.68, 95% CI 1.06–2.69, p = 0.024), fatigue/weakness (OR = 2.62, 95% CI 1.61–4.23, p < 0.001), and anemia (OR = 2.24, 95% CI 1.29–4.38, p = 0.006) compared with patients with SLE but not malignancy. Moreover, pulmonary hypertension and lung involvement were more prevalent in patients with SLE and malignancy (OR = 3.47, 95% CI 1.30–8.42, p = 0.007, and OR = 2.64, 95% CI 1.35–4.92, p = 0.003, respectively). Interestingly, there were no differences in mucocutaneous symptoms, joint involvement, pericardial effusion, pericarditis, hematological signs (except for anemia), blood ABO groups, Rh blood types (data not shown), kidney involvement, neurological abnormalities, Raynaud’s phenomenon, and lupoid hepatitis (p > 0.05 for all).
There were no differences in the autoantibody profile (Table 3), except for anticardiolipin antibodies in the IgG class, which were documented more frequently as positive in the non-malignancy-associated SLE group (p = 0.002). For example, anti-PR3 antibodies were examined in 144 (15.45%) out of 932 SLE patients, and anti-MPO antibodies were assessed in 151 (16.2%) out of 932 SLE patients. Anti-PR3 antibodies tested positive in only 7 (4.86%) of all SLE patients, whereas anti-MPO antibodies were positive in 14 (9.27% of all SLE patients) without statistically significant differences between SLE groups (p > 0.05 for both). Moreover, the malignancy-associated and non-malignancy-associated SLE groups did not differ in antithrombin activity, protein C activity, the free protein S level, factor VIII activity, factor V Leiden gene mutations, and G20210A prothrombin gene mutations (data not shown).
Throughout the median follow-up period of 14 years (ranges: 8–22 years), a total of 47 patients with SLE died, with 16 cases (5.28%) in the malignancy-associated SLE group and 31 cases (5.73%) in the non-malignancy SLE group (p = 0.76). Among the deceased, the most common causes of death were infections (6 cases, 37.5% vs. 4 cases, 12.9%) and SLE exacerbations (3 cases, 18.75% vs. 1 case, 3.23%), with no difference between the malignancy-associated and non-malignancy-associated SLE subgroups.
A higher frequency of internal comorbidities in patients with SLE and malignancy
The frequency of internal comorbidities was higher in the malignancy-associated SLE group compared with the non-malignancy-associated SLE group (Table 4). Specifically, patients with SLE and malignancy exhibited a higher occurrence of peripheral arterial obliterans disease (OR = 3.89, 95% CI 1.86–7.75, p < 0.001), heart failure (OR = 2.94, 95% CI 1.30–6.17, p = 0.005), diabetes mellitus (OR = 2.15, 95% CI 1.14–3.91, p = 0.011), hypothyroidism (OR = 2.08, 95% CI 1.29–3.34, p = 0.002), arterial hypertension (OR = 1.97, 95% CI 1.23–3.23, p = 0.003), and hypercholesterolemia (OR = 1.87, 95% CI 1.18-3.00, p = 0.006). Furthermore, myocardial infarction was diagnosed more frequently in the malignancy-associated SLE group compared with the non-malignancy-associated SLE group (OR = 3.08, 95% CI 1.41–6.30, p = 0.003). There were no significant differences in other arterial and venous thrombotic episodes between the groups.
Patients with SLE and malignancy were treated more aggressively before their malignancy diagnosis
Next, we delved into the various approaches to immunosuppressive therapy (Table 5). Steroids were the most commonly administered treatment for both the malignancy-associated and non-malignancy-associated SLE groups (94.51% and 96.18%, respectively, p = 0.62). The malignancy-associated SLE group was more frequently prescribed a more aggressive immunosuppressive regimen involving cyclophosphamide, which was utilized more often in this group (OR = 2.07, 95% CI 1.30–3.28, p = 0.002). In malignancy-associated SLE patients, the median cumulative dose of cyclophosphamide was 0 g (range: 0–2 g). Conversely, hydroxychloroquine was administered less frequently in the malignancy-associated SLE group compared with the non-malignancy-associated SLE group (OR = 0.62, 95% CI 0.39–0.98, p = 0.035).
Characteristics of malignancy in patients with SLE
The median age of patients at the time of the malignancy diagnosis was 56 years (48–63 years), with 18 malignancies diagnosed before SLE onset (median patient age 47.5 years [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] years), 9 at the same time of SLE diagnosis (median patient age 60 [54,55,56,57,58,59,60,61,62,63] years), and 62 reported after SLE diagnosis (median patient age 57.5 [50–65] years). The overall median duration between SLE onset and malignancy diagnosis was 9 (0–20) years. Notably, the distribution of non-hematologic and hematologic malignancies was comparable among the three abovementioned subgroups. Non-hematologic malignancies were more frequent than hematologic malignancies (n = 77, 83.7% vs. n = 15, 16.3%, p < 0.001). Detailed information is provided in Table 6. Among the non-hematologic malignancies, the most prevalent were breast cancer (n = 10), thyroid cancer (n = 8), and uterine cancer (n = 8). For the hematologic malignancies, the most common was non-Hodgkin lymphoma (n = 9).
Compared with patients with non-hematological malignancies, patients with hematological malignancies presented higher rates of LN diagnosed at the onset of SLE or during the first year of disease duration (36.67% vs. 15%, p = 0.02). Additionally, patients with hematological malignancies were diagnosed more frequently with monoclonal gammopathy of undetermined significance (MGUS) (60% vs. 1.61%, p < 0.001) and MAS (6.67% vs. 0%, p = 0.041). The treatment patterns also differed: Patients with hematological malignancies were treated more frequently with immunosuppressive drugs, including cyclosporine (20% vs. 3.33%, p = 0.009), mycophenolate mofetil (43.33% vs. 20%, p = 0.02), rituximab (16.67% vs. 1.67%, p = 0.007), and plasmapheresis (13.33% vs. 1.67%, p = 0.023). Furthermore, patients with hematological malignancies exhibited specific manifestations of SLE, including pericardial effusion (33.33% vs. 13.12%, p = 0.023), interstitial lung disease (20% vs. 4.84%, p = 0.022), and diffuse alveolar hemorrhage (6.67% vs. 0%, p = 0.04), more often that patients with non-hematological malignancies.
Patients with non-hematological malignancies manifested distinct features. This group was more often associated with SLE presenting with alopecia (29.03% vs. 6.67%, p = 0.015). Interestingly, skin vasculitis was observed only in patients with non-hematological malignancies (12.9%). Additionally, patients with non-hematological malignancies were treated more frequently with chloroquine or hydroxychloroquine (51.67% vs. 23.33%, p = 0.01).
Despite these differences, both groups showed similarities in the sex distribution, family history of autoimmune diseases, the mortality index, renal involvement in the course of SLE, the types of ANA and antineutrophil cytoplasmic antibodies (ANCA), and comorbidities, including thromboembolic episodes.
Analysis of the timing of malignancy diagnoses in relation to the timing of the SLE diagnosis revealed disparities between the groups. First, there were cases where malignancies were diagnosed prior to or concurrent with SLE. Second, there were instances where malignancies were diagnosed after SLE. In the first group, the median age at the onset of the first SLE symptoms was higher (54 vs. 37.5 years, p < 0.001), and the age at SLE onset was higher (59 vs. 39 years, p < 0.001). These subgroups did not differ in terms of LN frequency, but they did differ in the number of LN flares. For example, in the first group LN was diagnosed in 7 cases (25.93%) compared with 25 cases (40.32%) in the second group (p = 0.193). In turn, there were more LN flares in the second group (p = 0.018). However, the age of LN onset was higher in the first group compared to second one group (63 vs. 44.5 years, p < 0.001). In addition, the following were more common in the second group: myocardial infarction (0% vs. 17.74%, p = 0.019), treatment with azathioprine (7.41% vs. 53.33%, p < 0.001), malar rash (22.22% vs. 50%, p = 0.015), joint pain (74.07% vs. 95.16%, p = 0.004), and Raynaud’s phenomenon (7.41% vs. 27.42%, p = 0.034).
Discussion
We have provided comprehensive insights into the intricate relationship between SLE and malignancy, shedding light on various aspects, including demographic features, clinical manifestations, comorbidities, laboratory findings, and long-term outcomes. The observed prevalence of malignancies in the SLE cohort underscores the importance of understanding the intersection between these two complex conditions, because different types of malignancies occurred in almost 1 out of 10 patients from our SLE cohort.
Out of 932 patients with SLE, 9.87% had malignancy, with 7.61% of them had multiple malignancies. Patients with SLE and malignancy exhibited an older age at SLE onset and initial symptoms, with a significantly higher median age compared with patients with SLE but not malignancy. This observation is in line with previous reports [14, 15]. Moreover, both subgroups showed no differences in the sex distribution and time delay between symptom onset and diagnosis, with a similar disease duration between the groups. Approximately 70% of all malignancies were detected after a SLE diagnosis, consistent with the current knowledge [16]. However, it is worth highlighting that the risk of malignancy development in SLE is believed to be the highest during early SLE, especially in patients with a disease duration of < 1 year [16].
Interestingly, the pathogenesis of cancer development in patients with SLE is not fully understood [17]. Many studies have investigated the link between malignancy and SLE, pointing out the potential role of genetics and clinical or environmental factors in its development [18]. It has been postulated that an aberrant immune response in SLE not only contributes to the disease manifestations, but may also create an environment conducive for the development of malignancies. Moreover, changes in epigenetic regulations described in SLE might be linked with cancers, particularly regarding DNA methylation, histone modification, and non-coding messenger RNA (mRNA) activation. Furthermore, other mechanisms have been suggested, including high expression of a proliferation-inducing ligand (APRIL), a cytokine from the tumor necrosis factor ligand superfamily. APRIL might enable B cells in non-Hodgkin lymphoma to escape from apoptosis, favoring cancer development [19]. Furthermore, an increased level of interleukin(IL)-6 and IL-10 and the use of immunosuppressive therapy might also be related to the malignancy pathogenesis in SLE [20].
Several studies have consistently reported an increased risk of malignancy among patients with SLE. In a large cohort comprising 30,478 individuals with SLE, 1273 (4.18% of SLE cases) were diagnosed with malignancy. The prevalent malignancy types included breast cancer, lung cancer, colon/rectum cancer, and non-Hodgkin lymphoma. Notably, this cohort also exhibited occurrences of less common cancers such as valvular/vaginal cancer, which are infrequently observed in the general population [21]. However, our SLE cohort showed a higher incidence of malignancy, with almost 10% of patients being diagnosed, a higher rate compared with the study by Parikh-Patel et al. [21]. Furthermore, 7.61% of the enrolled patients with SLE and malignancy experienced at least two distinct malignancies. In a Korean population study [14], the estimated malignancy risk in patients with SLE was 1.45 (95% CI 0.74–2.16), with cervical cancer, non-Hodgkin lymphoma, and bladder cancer being the most frequently observed types. Cader et al. [22] evaluated 228 patients with SLE. Eight individuals were diagnosed with malignancy in its early stages, and they underwent radical treatment without signs of recurrence during the observation period.
Regarding hematological malignancies, our results align with the published data, with non-Hodgkin lymphoma being the most prevalent malignant disease in SLE [14, 21, 22]. In contrast, for non-hematological malignancies, besides breast, colorectal, uterine, and bladder cancers being the most frequently diagnosed; other frequently confirmed malignancies included thyroid, skin, and pancreatic cancers. Intriguingly, unlike the findings reported by Parikh-Patel et al. [21], lung cancer was quite rare in our SLE cohort: It was only diagnosed in two patients with SLE.
We did not observe any differences in a family history of various autoimmune diseases between the malignancy-associated and non-malignancy-associated SLE groups. These findings are consistent with the results reported by Bernatsky et al. [23]. While single case reports suggest a potential influence of a family history of SLE on malignancies in patients with SLE [24], our results are not consistent with these reports. Detailed information on familial factors in such a large SLE cohort, considering autoimmune disorders, is nearly absent in the current literature.
Constitutional manifestations, such as weight loss, fever, and fatigue/weakness, were more frequent in patients with SLE and malignancy. In clinical practice, it is crucial to distinguish these symptoms and to determine whether they are linked to a lupus flare, infection, or malignancy. Additionally, symptoms like anemia or pleural effusion may exist within the spectrum of SLE or indicate the onset of an oncological process. This poses a challenge for clinicians and practitioners [25, 26].
All patients in our SLE cohort exhibited positive ANA identified through IIF. ANA serve as a valuable serological marker for various autoimmune diseases, including SLE. It is important to note that they can also be present in the serum of patients with malignancies [27]. These data suggest that ANA could play a role in the pathogenesis of malignancy, as well as other premalignant diseases [27]. We confirmed the SLE diagnosis based on the EULAR/ACR criteria; therefore, we did not observe differences in the prevalence of ANA (based on IIF) between the SLE subgroups. In the study by Nisihara et al. [28], some patients with malignancy were positive for anti-RNP, anti-Sm, anti-Ro, and anti-La, which is consistent with our results. However, there were no differences in ANA types, as identified by immunoblot assay, between the malignancy-associated and non-malignancy-associated SLE groups. Intriguingly, Ladouceur et al. [19] highlighted the potential role of lupus-related anti-DNA antibodies in reducing the risk of breast cancer by inhibiting DNA repair. Moreover, our findings align with the study by Shah et al. [29], who observed a decreased risk of breast cancer in cases where anti-dsDNA or anti-La antibodies were positive.
Another crucial issue that requires discussion is the association between ANCA and malignancy. We did not observe differences in the frequencies of anti-PR3 and anti-MPO antibodies between the malignancy-associated and non-malignancy-associated SLE groups. Conversely, the current literature indicates that ANCA are produced in malignancy and are not necessarily related to autoimmune vasculitis, as commonly believed [30]. Additionally, ANCA might be associated with a poor prognosis for some cancers, such as Ewing’s sarcoma and cutaneous melanoma [30]. This discrepancy may be linked to the interference of ANA in the differentiation between the perinuclear ANCA (p-ANCA) and cytoplasmic ANCA (c-ANCA) patterns [31], as well as the utilization of ELISAs in a relatively small study group.
Of particular interest concerning antiphospholipid antibodies (aPLA) is the higher incidence of anticardiolipin antibodies of the IgG class in the non-malignancy-associated SLE group. This finding is in contrast to the data presented by Armas et al. [32]. Moreover, the presence of aPLA has been reported in conjunction with various solid and hematologic malignancies [33, 34]. So far, patients with malignancies and aPLA are characterized by a higher incidence of thromboembolic events, but we did not confirm such a relationship in our study [35]. We observed a higher incidence of myocardial infarction in the malignancy-associated SLE group, but without an increased frequency of aPLA in this group. Therefore, myocardial infarction may be a consequence of other factors, including comorbidities associated with SLE.
Despite increased recognition of SLE with more sensitive diagnostic tests, the inclusion of milder cases, earlier diagnosis or treatment, increasingly judicious therapy, and prompt treatment of complications, patients with SLE still have mortality rates that are up to 5.3 times higher than that of the general population [36,37,38,39,40,41].
Interestingly, the malignancy process did not impact the increasing the mortality risk in our SLE cohort, a finding that is in line with data presented by Yurkovich et al. [42]. These comparable mortality rates with different times of malignancy onset in patients with SLE underscore the persistent need for regular oncological screening in these patients.
Another intriguing finding from our study is the increased incidence of arterial hypertension, diabetes mellitus, heart failure, and hypercholesterolemia in patients with SLE and malignancy. Importantly, SLE and malignancy share common risk factors that accumulate over a lifetime, such as smoking, a sedentary lifestyle, and dietary habits [43]. Metabolic syndrome, which encompasses all these risk factors, is significantly associated with the risk of obesity-related malignancies, particularly pancreatic, postmenopausal breast, endometrial, and colorectal cancers [43, 44]. Inflammation, which is linked to atherosclerosis and diabetes mellitus, also plays a role in carcinogenesis, but this relationship is bidirectional. Conversely, malignancy treatment can induce endothelial damage, further exacerbating atherosclerosis [45]. Endothelial dysfunction has been investigated in other connective tissue diseases and appears to be a hallmark of these conditions [46,47,48,49]. Furthermore, molecules that are involved in lipid metabolism, endothelial function, regulation of autophagy, and metastatic process in malignancies have been identified [50,51,52]. This issue warrants further investigation and underscores the complexity of metabolic pathways and their impact on overall health.
Finally, it is worth discussing the impact of immunosuppressive drugs on oncogenesis in the SLE cohort. Cyclophosphamide is an alkylating agent used as an immunosuppressant and chemotherapeutic. It was administered more often in the malignancy-associated SLE group compared with the non-malignancy-associated SLE group. Despite these properties, it has been found to be carcinogenic, and the risk increases with the length of exposure. The most common malignancies associated with this agent are bladder cancer, skin cancer, and secondary acute leukemia (preceded by myelodysplastic syndrome) [53]. However, researchers have reported conflicting data on this matter in patients with SLE. The findings from a Swedish registry indicated that cases of myeloid leukemia in patients with SLE were not related to cyclophosphamide exposure [54], mirroring the observations reported by Guo et al. [55], who found no discernible associations between drug exposure and malignancy risk. Conversely, Bernatsky et al. [56] suggested an increased risk of hematological malignancies in patients with SLE following exposure to immunosuppressants (hazard ratio [HR] 2.29, 95% CI 1.02–5.15) with cyclophosphamide driving the risk (drug-specific HR for exposure of 3.55, 95% CI 0.94–13.37), although associations with other immunosuppressants were not evident. Another study indicated an increased risk of non-melanoma skin cancer with cyclophosphamide (adjusted HR 15.3, 95% CI 3.03–77.5), but no associations with other cancer types [57]. Notably, the cumulative dose of cyclophosphamide appears to be of particular concern [14].
The protective effect of hydroxychloroquine has been suggested previously, and our study aligns with these findings. Antimalarial drugs have been shown to decrease the risk of breast and non-melanoma skin cancer in patients with SLE [57]. Another study found that hydroxychloroquine was associated with decreased odds of cancer (OR 0.417, 95% CI 0.220–0.791) [55]. Ruis-Irastaroze et al. [58] evaluated the impact of antimalarials on cancer risk in patients with SLE, revealing an 85% reduction in cancer risk among those treated with antimalarials (HR 0.15, 95% CI 0.02–0.99). Hydroxychloroquine has also garnered attention in cancer treatment. Evidence suggests that it may enhance the anti-tumoral response in hormone-positive breast cancer when combined with tamoxifen or faslodex, agents that block estrogen receptors [59]. Furthermore, a phase I trial tested the combination of hydroxychloroquine and erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, in EGFR-mutant non-small cell lung cancer; it demonstrated preliminary activity and acceptable tolerability [60]. Additionally, a randomized, double-blind, placebo-controlled trial was performed in patients with glioblastoma multiforme who were treated with standard chemoradiotherapy with chloroquine or placebo after tumor resection. Although the HR did not reach statistical significance (0.52, 95% CI 0.21–1.26, p = 0.139), the median survival was 24 months in the chloroquine-treated cohort versus 11 months for the controls [61]. Several potential explanations for these observations have been proposed. One possible anti-tumoral mechanism involved the promotion of autophagy, which plays an important role in cancer cell invasion [62], providing the rationale for combining antimalarials with DNA-damaging chemotherapy [63]. Chloroquine has also been shown to inhibit telomerase, which is involved in the unlimited replication of cancer cells [61], and to increase the synthesis of TP53, a protein whose expression increases in response to genotoxic stimuli or environmental stress [64].
Study limitations
Our study is subject to several limitations. The retrospective design introduces potential biases related to reliance on historical medical records. The single-center approach raises concerns about external validity, as patient demographics and practices may vary across different settings. The observational nature precludes causal inferences, and unaccounted confounders may influence the outcomes. Retrospective data collection might result in incomplete information, impacting the accuracy of our analysis. Our focus on specific features may have led us to overlook other relevant variables. Addressing these limitations is crucial for interpreting our results and guiding future research in understanding the intricate relationship between SLE and malignancy.
Conclusions
The comprehensive analysis of demographic features, clinical manifestations, and long-term outcomes in patients with SLE and with or without malignancy provides valuable insights into the multifaceted nature of these conditions in a real-world setting. Our findings underscore the clinical complexity in individuals with SLE who develop malignancy, revealing distinct patterns of comorbidities, immunosuppressive treatment, and clinical manifestations.
References
Kuhn A, Bonsmann G, Anders H-J, Herzer P, Tenbrock K, Schneider M (2015) The Diagnosis and Treatment of Systemic Lupus Erythematosus, Deutsches Ärzteblatt international, Jun. https://doi.org/10.3238/arztebl.2015.0423
Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT (Jan. 2021) Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis 80(1):14–25. https://doi.org/10.1136/annrheumdis-2020-218272
Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y (2018) The risks of cancer development in systemic lupus erythematosus (SLE) patients: a systematic review and meta-analysis, Arthritis Res Ther, vol. 20, no. 1, p. 270, Dec. https://doi.org/10.1186/s13075-018-1760-3
Clarke AE et al (2021) Dec., Risk of malignancy in patients with systemic lupus erythematosus: Systematic review and meta-analysis, Seminars in Arthritis and Rheumatism, vol. 51, no. 6, pp. 1230–1241, https://doi.org/10.1016/j.semarthrit.2021.09.009
Bernatsky S (2005) Non-Hodgkin’s lymphoma in systemic lupus erythematosus, Annals of the Rheumatic Diseases, vol. 64, no. 10, pp. 1507–1509, Oct. https://doi.org/10.1136/ard.2004.034504
Gayed M, Bernatsky S, Ramsey-Goldman R, Clarke A, Gordon C (May 2009) Lupus and cancer. Lupus 18:479–485. https://doi.org/10.1177/0961203309102556
Bernatsky S, Ramsey-Goldman R, Foulkes WD, Gordon C, Clarke AE (2011) Breast, ovarian, and endometrial malignancies in systemic lupus erythematosus: a meta-analysis, Br J Cancer, vol. 104, no. 9, pp. 1478–1481, Apr. https://doi.org/10.1038/bjc.2011.115
Hansen JE et al (Oct. 2012) Targeting Cancer with a Lupus Autoantibody. Sci Transl Med 4(157). https://doi.org/10.1126/scitranslmed.3004385
Zhang M, Wang Y, Wang Y, Bai Y, Gu D (May 2022) Association between systemic Lupus Erythematosus and Cancer Morbidity and Mortality: findings from Cohort studies. Front Oncol 12:860794. https://doi.org/10.3389/fonc.2022.860794
Kariniemi S, Rantalaiho V, Virta LJ, Kautiainen H, Puolakka K, Elfving P (2022) Malignancies among newly diagnosed systemic lupus erythematosus patients and their survival, Lupus, vol. 31, no. 14, pp. 1750–1758, Dec. https://doi.org/10.1177/09612033221131501
Aringer M (2019) EULAR/ACR classification criteria for SLE, Seminars in Arthritis and Rheumatism, vol. 49, no. 3, pp. S14–S17, Dec. https://doi.org/10.1016/j.semarthrit.2019.09.009
Kosałka-Węgiel J et al (2024) Clinical and laboratory characteristics of early-onset and delayed-onset lupus nephritis patients: a single-center retrospective study. Rheumatol Int Mar. https://doi.org/10.1007/s00296-024-05579-4
Levey AS, Titan SM, Powe NR, Coresh J, Inker LA, Kidney Disease, Race, and, Estimation GFR (2020) CJASN, vol. 15, no. 8, pp. 1203–1212, Aug. https://doi.org/10.2215/CJN.12791019
Kang KY et al (Apr. 2010) Incidence of cancer among female patients with systemic lupus erythematosus in Korea. Clin Rheumatol 29(4):381–388. https://doi.org/10.1007/s10067-009-1332-7
Nived O, Bengtsson A, Jönsen A, Sturfelt G, Olsson H (2001) Malignancies during follow-up in an epidemiologically defined systemic lupus erythematosus inception cohort in southern Sweden, Lupus, vol. 10, no. 7, pp. 500–504, Jul. https://doi.org/10.1191/096120301678416079
Bernatsky S et al (May 2005) An international cohort study of cancer in systemic lupus erythematosus. Arthr Rhuem 52(5):1481–1490. https://doi.org/10.1002/art.21029
Hardenbergh D, Molina E, Naik R, Geetha D, Chaturvedi S, Timlin H (2022) Factors mediating cancer risk in systemic lupus erythematosus, Lupus, vol. 31, no. 11, pp. 1285–1295, Oct. https://doi.org/10.1177/09612033221122163
Ladouceur A, Clarke AE, Ramsey-Goldman R, Bernatsky S (2019) Malignancies in systemic lupus erythematosus: an update, Current Opinion in Rheumatology, vol. 31, no. 6, pp. 678–681, Nov. https://doi.org/10.1097/BOR.0000000000000648
Ladouceur A et al (Aug. 2020) Cancer and systemic Lupus Erythematosus. Rheumatic Disease Clin North Am 46(3):533–550. https://doi.org/10.1016/j.rdc.2020.05.005
Hsu C-Y et al (Dec. 2016) Cumulative immunosuppressant exposure is associated with diversified cancer risk among 14 832 patients with systemic lupus erythematosus: a nested case–control study. Rheumatology kew457. https://doi.org/10.1093/rheumatology/kew457
Parikh-Patel A, White RH, Allen M, Cress R (2008) Cancer risk in a cohort of patients with systemic lupus erythematosus (SLE) in California, Cancer Causes Control, vol. 19, no. 8, pp. 887–894, Oct. https://doi.org/10.1007/s10552-008-9151-8
Cader RA, Mei Yee AK, Yassin A, Ahmad I, Haron SN (2018) Malignancy in Systemic Lupus Erythematosus (SLE) Patients, Asian Pac J Cancer Prev, vol. 19, no. 12, pp. 3551–3555, Dec. https://doi.org/10.31557/APJCP.2018.19.12.3551
Bernatsky S et al (2006) Oct., Malignancy prevalence in the first-degree relatives of persons with systemic lupus erythematous: a pilot study, Lupus, vol. 15, no. 10, pp. 695–696, https://doi.org/10.1177/0961203306072424
Ando B, Nawata H, Umeda F, Ibayashi H (1986) A patient with familial systemic lupus erythematosus associated with gastric cancer and a family study of HLA, Fukuoka Igaku Zasshi, vol. 77, no. 9, pp. 526–531, Sep
Jung J-Y, Suh C-H (May 2017) Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J Intern Med 32(3):429–438. https://doi.org/10.3904/kjim.2016.234
Rao M, Mikdashi J (2023) A Framework to Overcome Challenges in the Management of Infections in Patients with Systemic Lupus Erythematosus, OARRR, vol. Volume 15, pp. 125–137, Jul. https://doi.org/10.2147/OARRR.S295036
Vlagea A et al (Jul. 2018) Antinuclear antibodies and cancer: a literature review. Crit Rev Oncol/Hematol 127:42–49. https://doi.org/10.1016/j.critrevonc.2018.05.002
Nisihara R, Machoski MCC, Neppel A, Maestri CA, Messias-Reason I, Skare TL (2018) Anti-nuclear antibodies in patients with breast cancer, Clinical and Experimental Immunology, vol. 193, no. 2, pp. 178–182, Jul. https://doi.org/10.1111/cei.13136
Shah AA et al (Dec. 2021) Association of systemic lupus erythematosus autoantibody diversity with breast cancer protection. Arthritis Res Ther 23(1):64. https://doi.org/10.1186/s13075-021-02449-3
Scandolara TB, Panis C (May 2020) Neutrophil traps, anti-myeloperoxidase antibodies and cancer: are they linked? Immunol Lett 221:33–38. https://doi.org/10.1016/j.imlet.2020.02.011
Romero-Sánchez C et al (2020) Nov., Frecuencia de ANCA positivos en una población con síntomas clínicos sugestivos de enfermedad autoinmune y la interferencia de ANA en su interpretación, Reumatología Clínica, vol. 16, no. 6, pp. 473–479, https://doi.org/10.1016/j.reuma.2018.09.007
Armas JB et al (2000) Anticardiolipin and antinuclear antibodies in cancer patients–a case control study. Clin Exp Rheumatol 18(2):227–232
Pugliese L, Bernardini I, Pacifico E, Viola-Magni M, Albi E (2006) Antiphospholipid Antibodies in Patients with Cancer, Int J Immunopathol Pharmacol, vol. 19, no. 4, pp. 879–888, Oct. https://doi.org/10.1177/039463200601900417
Gómez-Puerta JA et al (2006) Apr., Antiphospholipid Antibodies Associated with Malignancies: Clinical and Pathological Characteristics of 120 Patients, Seminars in Arthritis and Rheumatism, vol. 35, no. 5, pp. 322–332, https://doi.org/10.1016/j.semarthrit.2005.07.003
Pham C, Shen Y-M (2008) Antiphospholipid Antibodies and Malignancy, Hematology/Oncology Clinics of North America, vol. 22, no. 1, pp. 121–130, Feb. https://doi.org/10.1016/j.hoc.2007.10.004
Seleznick MJ, Fries JF (Oct. 1991) Variables associated with decreased survival in systemic lupus erythematosus. Semin Arthritis Rheum 21(2):73–80. https://doi.org/10.1016/0049-0172(91)90040-7
Ward MM, Pyun E, Studenski S (1996) Mortality risks associated with specific clinical manifestations of systemic lupus erythematosus, Arch Intern Med, vol. 156, no. 12, pp. 1337–1344, Jun
Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME (Aug. 2004) Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmun rev 3(6):423–453. https://doi.org/10.1016/j.autrev.2004.04.002
Singh RR, Yen EY (2018) SLE mortality remains disproportionately high, despite improvements over the last decade, Lupus, vol. 27, no. 10, pp. 1577–1581, Sep. https://doi.org/10.1177/0961203318786436
Trager J, Ward MM (2001) Mortality and causes of death in systemic lupus erythematosus, Curr Opin Rheumatol, vol. 13, no. 5, pp. 345–351, Sep. https://doi.org/10.1097/00002281-200109000-00002
Cervera R et al (2003) Sep., Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients, Medicine (Baltimore), vol. 82, no. 5, pp. 299–308, https://doi.org/10.1097/01.md.0000091181.93122.55
Yurkovich M, Vostretsova K, Chen W, Aviña-Zubieta JA (2014) Overall and Cause‐Specific Mortality in Patients With Systemic Lupus Erythematosus: A Meta‐Analysis of Observational Studies, Arthritis Care & Research, vol. 66, no. 4, pp. 608–616, Apr. https://doi.org/10.1002/acr.22173
Raposeiras Roubín S, Cordero A (2019) The Two-way Relationship Between Cancer and Atherosclerosis, Revista Española de Cardiología (English Edition), vol. 72, no. 6, pp. 487–494, Jun. https://doi.org/10.1016/j.rec.2018.12.010
Sun M et al (2023) Apr., Metabolically (un)healthy obesity and risk of obesity-related cancers: a pooled study, JNCI: Journal of the National Cancer Institute, vol. 115, no. 4, pp. 456–467, https://doi.org/10.1093/jnci/djad008
Pacholczak R, Dropiński J, Walocha J, Musiał J (Nov. 2018) Anti-cancer agents and endothelium. Oncol Clin Pract 14(5):249–256. https://doi.org/10.5603/OCP.2018.0032
Kuszmiersz P et al (2021) Jul., Thrombin generation potential is enhanced in systemic sclerosis: impact of selected endothelial biomarkers, Clinical and Experimental Rheumatology, vol. 39, no. 4, pp. 13–19, https://doi.org/10.55563/clinexprheumatol/d03dnc
Pacholczak-Madej R et al (2020) Endothelial dysfunction in patients with systemic sclerosis, pdia, vol. 37, no. 4, pp. 495–502, https://doi.org/10.5114/ada.2019.83501
Pacholczak R et al (Feb. 2019) Endothelial dysfunction in patients with eosinophilic granulomatosis with polyangiitis. Clin Rheumatol 38(2):417–424. https://doi.org/10.1007/s10067-018-4253-5
Pacholczak R et al (Aug. 2018) Endothelial dysfunction in patients with granulomatosis with polyangiitis: a case–control study. Rheumatol Int 38(8):1521–1530. https://doi.org/10.1007/s00296-018-4061-x
Gupta A, Das D, Taneja R (Mar. 2024) Targeting dysregulated lipid metabolism in Cancer with pharmacological inhibitors. Cancers 16:1313. https://doi.org/10.3390/cancers16071313
Filippini A, Tamagnone L, D’Alessio A (1929) Endothelial Cell Metabolism in Vascular Functions, Cancers, vol. 14, no. 8, p. Apr. 2022, https://doi.org/10.3390/cancers14081929
Strippoli R et al (Feb. 2024) Contribution of autophagy to epithelial mesenchymal transition induction during Cancer Progression. Cancers 16(4):807. https://doi.org/10.3390/cancers16040807
Emadi A, Jones RJ, Brodsky RA (2009) Cyclophosphamide and cancer: golden anniversary, Nat Rev Clin Oncol, vol. 6, no. 11, pp. 638–647, Nov. https://doi.org/10.1038/nrclinonc.2009.146
Lofstrom B, Backlin C, Sundstrom C, Hellstrom-Lindberg E, Ekbom A, Lundberg IE (2009) Myeloid leukaemia in systemic lupus erythematosus–a nested case-control study based on Swedish registers, Rheumatology, vol. 48, no. 10, pp. 1222–1226, Oct. https://doi.org/10.1093/rheumatology/kep204
Guo J, Ren Z, Li J, Li T, Liu S, Yu Z (2020) The relationship between cancer and medication exposure in patients with systemic lupus erythematosus: a nested case-control study, Arthritis Res Ther, vol. 22, no. 1, p. 159, Dec. https://doi.org/10.1186/s13075-020-02228-6
Bernatsky S et al (2008) Jan., The relationship between cancer and medication exposures in systemic lupus erythaematosus: a case-cohort study, Annals of the Rheumatic Diseases, vol. 67, no. 1, pp. 74–79, https://doi.org/10.1136/ard.2006.069039
Bernatsky S et al (2021) Dec., Cancer Risk in a Large Inception Systemic Lupus Erythematosus Cohort: Effects of Demographic Characteristics, Smoking, and Medications, Arthritis Care & Research, vol. 73, no. 12, pp. 1789–1795, https://doi.org/10.1002/acr.24425
Ruiz-Irastorza G et al (2007) Jan., Antimalarials may influence the risk of malignancy in systemic lupus erythematosus, Annals of the Rheumatic Diseases, vol. 66, no. 6, pp. 815–817, https://doi.org/10.1136/ard.2006.067777
Cook KL et al (2014) Jun., Chloroquine Inhibits Autophagy to Potentiate Antiestrogen Responsiveness in ER + Breast Cancer, Clinical Cancer Research, vol. 20, no. 12, pp. 3222–3232, https://doi.org/10.1158/1078-0432.CCR-13-3227
Goldberg SB et al (Oct. 2012) A phase I study of Erlotinib and Hydroxychloroquine in Advanced non–small-cell Lung Cancer. J Thorac Oncol 7(10):1602–1608. https://doi.org/10.1097/JTO.0b013e318262de4a
Sotelo J, Briceño E, López-González MA (Mar. 2006) Adding chloroquine to Conventional Treatment for Glioblastoma Multiforme: a Randomized, Double-Blind, placebo-controlled trial. Ann Intern Med 144(5):337. https://doi.org/10.7326/0003-4819-144-5-200603070-00008
Li J et al (2013) Jun., Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial–mesenchymal transition, Carcinogenesis, vol. 34, no. 6, pp. 1343–1351, https://doi.org/10.1093/carcin/bgt063
Pan Y et al (May 2011) Targeting Autophagy Augments in Vitro and in vivo antimyeloma activity of DNA-Damaging chemotherapy. Clin Cancer Res 17(10):3248–3258. https://doi.org/10.1158/1078-0432.CCR-10-0890
Sohn TA, Bansal R, Su GH, Murphy KM, Kern SE (2002) High-throughput measurement of the Tp53 response to anticancer drugs and random compounds using a stably integrated Tp53-responsive luciferase reporter, Carcinogenesis, vol. 23, no. 6, pp. 949–958, Jun. https://doi.org/10.1093/carcin/23.6.949
Funding
This work was supported by the Research Grant of Jagiellonian University Medical College No. N41/DBS/000936 (to J.K.-W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
not declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kosałka-Węgiel, J., Pacholczak-Madej, R., Dziedzic, R. et al. Malignancy in systemic lupus erythematosus: relation to disease characteristics in 92 patients – a single center retrospective study. Rheumatol Int (2024). https://doi.org/10.1007/s00296-024-05623-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00296-024-05623-3