Abstract
The study aimed to explore the experience of coronavirus disease-2019 (COVID-19) infection and vaccine adverse events (AEs) among rheumatologists. A validated questionnaire was distributed as a Google form to rheumatologists across the country via social networking sites from late December 2021 till early January 2022. The questionnaire included questions regarding participants' socio-demographic details, COVID-19 infection and vaccination details with special emphasis on AEs. Out of 246 responses, 228 were valid. 200 (81.3%) responders had received the vaccine. The mean age of the 228 participants was 37.9 ± 8.5 years, 196 were females and 32 males (F:M 6.1:1) from 18 governorates across the country. Comorbidities were present in 54 subjects (27%). There was a history of highly suspicious or confirmed COVID-19 infection in 66.7% that were all managed at home. The COVID-19 vaccine was received by 200 and a booster dose of 18.5%. Obesity and musculoskeletal involvement co-morbidities were present only in those with AEs (9.1% and 5.5% respectively). AEs were present in 82%; 66.7% had injection-site tenderness, 50% fatigue, 35.5% fever, 15% chills, 42.5% myalgia, 14.5% arthralgia, 8% low back pain, headache 31%, dizziness 10%, sleepliness 16% and 15% developed post-vaccine. There were no differences according to the geolocation regarding the occurrence of COVID-19 infection (p = 0.19) or AEs post-vaccine (p = 0.58). The adverse events were mostly mild to moderate and tolerable which makes this work in agreement with other studies that support the broad safety of the vaccine in favor of the global benefit from mass vaccination.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) has remarkably lead to social and economic damage and a notable impact on public health, and vaccines are being developed to fight the disease [1]. Rheumatologists in Egypt are enormously expanding and mastering the tools that aid them in enhancing the management of rheumatic diseases and there is a need to boost their practice in spite the opportunities and challenges [2]. Health care workers are facing an increasing threat while confronting the COVID-19 pandemic [3]. COVID-19 is a forerunning cause claiming the lives of healthcare professionals worldwide [4]. The innovation of the vaccine is expected to preserve public health and safety and remains to be challenging even if an effective vaccine is developed [3].
The world is desperate for a cure as humanity is suffering from the COVID-19 pandemic and its consequences [5]. Throughout the height of the COVID-19 pandemic, there was immense relief with the worldwide mass rollout of the COVID-19 vaccines. While they have proven to be safe and effective, the ongoing appearance of side effects of the vaccines has undermined public trust and, although exceptional, can lead to considerable morbidity and mortality [6]. Unfortunately, the shorter production time has raised doubts about the safety of these vaccines [5].
In the previous Vaxurvey study on the acceptability of the COIVD-19 vaccine, results were modest and alarming to Egyptian health authorities to stir further modes to reduce the levels of vaccine hesitancy. And it was recommended to investigate any possible side effects [7]. It has been noticed that those with clinical conditions or immunosuppression, the immune response to COVID-19 vaccine was steady with high levels of efficiency. Enhancing the coverage in immunosuppressed subjects and the prioritizing this group for third doses could be supported [8].
The most serious adverse event of some COVID-19 vaccines is the occurrence of vaccine-induced immune thrombocytopenia and thrombosis that is a frequently fatal complication seen more in younger people and women [6]. Whilst there is a lack of long-term safety and efficacy data of COVID-19 vaccination in patients with autoimmune diseases, the existing evidence robustly suggests that the benefits of vaccination outweigh the risks of adverse effects and disease flares [9].
During the pandemic, rheumatologists agreed to their key emerging frontline role in treating COVID-19. Prior survey results highlight the critical importance of concerns related to vaccine safety and efficacy for people with rheumatic diseases, which appear to have persisted after widespread vaccination. Willingness of the rheumatologist to get vaccinated and their confidence on the COVID-19 vaccine safety and tolerability would influence the decision of their RD patients to receive COVID-19 vaccine too. Patients are willing to receive the COVID-19 vaccine, hence, rheumatologists should be prepared with the proper information before counseling their patients. Thus, the study aimed to explore the experience of COVID-19 infection and vaccine adverse events among rheumatologists.
Methods
A comprehensive self-report open e-survey was structured to appraise the COVID-19 infection and vaccine safety in Rheumatologists in Egypt. The questionnaire was provided on a cloud-based website- google form, and scrutinized by three experts. The survey included a total of 32 questions; 29 questions designed to evaluate previous COVID-19 infection, vaccination status, and adverse events (AEs) following vaccine administration. Rheumatologists from 15 universities and institutions conducted the e-survey on colleagues in various governorates that are willing to participate. Moreover, the survey was shared on relevant social media platforms on WhatsApp and Facebook. The survey was accepting responses for 2 weeks from late December 2021 to early January 2022. The data were then analyzed.
Survey design
The questionnaire featured 29 questions, covering certain areas comprising the contact email (1 question), basic/demographic features (9 questions) (Country, city, country of vaccination, age, gender, marital status, specialty, affiliation, professional rank), co-morbidities/chronic diseases (3 questions), previous COVID infection, its diagnosis and management (3 questions), vaccination (willingness, status, type, booster) (4 questions) and vaccine adverse effects and outcome (9 questions). There were 10 multiple choice questions requiring a single answer option, 7 questions check box list with multiple answer options selection, 11questions with short answers (if applicable) and 1 question drop-down list. Choices were closed ended for (18 questions), while the short answers questions “other or please specify” option in 11 questions, where deemed appropriate. All the survey questions were adapted to one screen page (Supplementary Table 1). Participants were able to review and change their answers before their submission. The survey was designed by TG and revised by HF and NH.
Pilot testing and validation
For pretest validation of the survey, a pilot test was run three rheumatologists (TG, HF and NH) and a revision of the survey design, content, terminologies, comprehension and easiness to conduct. The average survey time was 3 minutes. The respondents could not change the answers after submission. All questions, except the short answers, were mandatory.
Population selection
There was no particular sampling technique used, and all those who agreed to participate were included in the survey. The survey was prepared and presented in a google form and sent out to the fellows and colleagues willing to share. Duplicate, invalid or incomplete responses were excluded. Informed consent was provided from all subjects involved in the study and no incentives were offered for survey completion. The study was conducted in accordance with the guidelines of the declaration of Helsinki. It was approved by the Scientific Research Ethics Committee numbered (R215), in its session (90) on 9/1/2022. The survey was in accordance with the Checklist for Reporting Results of Internet E-surveys [10].
Data handling and confidentiality
Email address checks were done and duplicate responses from a single respondent were removed. Non- Egyptian participants were excluded. The survey was in part anonymized with emails being the only linked identifiers. Data handling was completely anonymous with the email remaining with the corresponding author.
Statistical analysis
Data was downloaded from google forms into Microsoft Excel for analysis. Descriptive statistics were used; data were presented as number (percentage) or mean±SD (range). Comparisons were done by Mann-Whitney and one-way analysis of variance (ANOVA) tests. Open-ended responses in the “Others (please specify)” category were manually allocated to existent categories, and a new category was listed when the responses did not fit into an existent category. P-values were significant at p < 0.05
Results
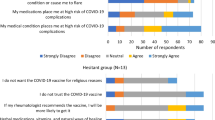
The study included 246 responses to the survey from Egyptian rheumatologists across the country. Out of which 228 were valid, 200 (81.3%) responders had received the COVID-19 vaccine, while 28 (18.7%) did not receive the vaccine. The mean age of the 228 participants was 37.9 ± 8.5 years and they were 196 females and 32 males (F:M 6.1:1). 174 were married, 46 single, 5 divorced and 3 widows. Hundred and fifty five were university staff members, 43 working at ministry of health hospitals, 25 from teaching hospitals and 3 from the military armed forces hospitals while 31 had an additional private practice. 18 were internists with a rheumatology practice. They were 47 residents, 32 specialists, 13 consultants, 36 assistant lecturers, 34 lecturers, 36 assistant professors, and 27 professors, and 3 fellows. The participants were from the following 18 north-south governorates: Alexandria (4.8%), Damiette (0.9%), Kafr El Sheikh (0.9%), Beheira (0.9%), Dakahlia (6.6%), Gharbia (1.8%), Sharkia (1.3%), Ismailia (0.9%), Menoufiya (0.9%), Kalyoubia (2.2%), Great Cairo (Cairo/Giza/Helwan/6th October) (31.1%), Fayoum (3.9%), Beni-Suef (14.5%), Minia (12.7%), Assuit (13.2%), Sohag (2.2%), Luxor (0.4%) and Aswan (0.9%). 15 received the vaccine abroad (1 USA, 1 Kuwait, 2 UAE and 11 KSA). Regarding comorbidities and chronic diseases condition were present in 54 subjects (27%);10 were diabetic, 21 hypertensive, 16 obese, 10 had thyroid dysfunction, 9 musculoskeletal disorders, 4 with chest problems, 2 cardiac dysfunction, and one each with multiple sclerosis (MS), psoriasis, primary anti-phospholipid syndrome, inflammatory bowel syndrome (IBS), dyslipidemia, sinusitis, atopy, migraine, depression, familial Mediterranean fever (FMF) and another with cancer (chronic lymphocytic leukemia: CLL) on chemotherapy.
The history of COVID-19 infection frequency, mode and vaccination of the studied subjects are presented in Table 1. All were managed at home. According to the professional rank, 70.7% of the residents, 79.3% specialists, 27.3% consultants, 89.3% assistant lecturers, 58.1% lecturers, 62.5% assistant professors, 52% professors and 33.3% of fellows had a history of previous COVID-19 (p = 0.002).
The adverse events related to the COVID-19 vaccine are presented in Table 2. Among the 164 reporting AEs, 31 had only injection site tenderness and one had swelling. The participants that developed COVID-19 infection after vaccination were all managed at home. Post-vaccination, laboratory investigations were performed by 27 and all were normal except for four; one with lymphopenia, increased CRP and ferritin, two with an elevated CRP, one with elevated D-dimer and another with an elevated LDH. Seven rheumatologists performed a CT scan post-vaccination; one with the atelectatic band and a pneumatic patch, one with COVID-19 Reporting and Data System (CORADS) CORADS4 (very highly suspicious of COVID-19), one with CORADS5 (typical findings of COVID-19) and another with bilateral pneumonia. AEs post-vaccination was significantly present in 48% after 1st dose, 16.5% after 2nd dose and in 16% after both doses (p < 0.0001). There were no significant differences between females and males in all COVID-19 related AEs except for dizziness as which was more prevalent in females (p = 0.04).
On comparing those who had any AEs and those not, findings are presented in Table 3. There were no differences according to the geolocation regarding the occurrence of COVID-19 infection (p=0.19) or the development of AEs after the vaccine (p = 0.58). The frequency of having COVID-19 infection, having AEs or COVID-19 post vaccine was comparable between those receiving the vaccine inside Egypt or abroad (p = 0.15, p = 0.11 and p = 0.23, respectively).
Discussion
The mass vaccination represents a turning point in the global battle against the COVID-19 pandemic, an unprecedented challenge for physicians, healthcare professionals, health systems and pharmaceutical companies. However, accumulating real-life data confirm the favorable overall safety profile of the vaccines [11]. Yet, those who suffered from mild or severe AEs should be carefully considered by refining the quality of the vaccines to approach a nearly zero side effect. To our knowledge, no prior studies have examined the perceptions and experience of COVID-19 vaccine safety and tolerability among rheumatologists.
In this work, Rheumatologists reported a previous infection with COVID-19, confirmed or highly suspicious in 66.7%. In a study from Ethiopia on health care workers, 13.2% were previously infected and 32.5% not sure. In fact 53.2% of their respondents think that the vaccine will not be effective in preventing the transmission of COVID-19 and only 50% received the full dose of the vaccine [3]. During the pandemic, rheumatic disease cases requiring admission were dealt with by several modified strategies [12].
In this work, 200 (87.7%) Egyptian rheumatologists were vaccinated. Timely vaccination may avert complications and morbidity yet may lead to unforeseen outcomes in some special clinical populations [1]. Increasing the number of people vaccinated against COVID-19 is considered one of the most effective strategies to control the pandemic. Adverse events after vaccination have become a challenge; myocarditis and pericarditis are frequently reported following receipt of messenger RNA (mRNA) vaccines, including BNT162b2 (Pfizer vaccine) and mRNA-1273 (Moderna vaccine) and neurological AEs have also been reported [13]. In Africa, the low vaccine coverage and the common vaccine hesitancy undermine efforts to fight the COVID-19 pandemic [14].
One of the participants had CLL and did not have any AEs post-vaccine. It has been suggested that patients with hematologic malignancy, likely do not elicit robust immunologic responses following COVID-19 vaccination [15]. A third boosting vaccine dosage should be considered for these patients [16].
In the present work, 164 (82%) had mild to moderate AEs and 31 (15.5%) had only injection site tenderness and none required hospitalization and were managed at home. 94.3% of AE following immunization against COVID-19 in Australia were non-serious [17]. In this work, there was a tendency that AEs were more in females. The significant predictors of side effects were the female gender and a history of allergies [5].
Injection site tenderness was present in 66.5% and swelling in one participant. Pfizer-BioNTech BNT162b2 most common side effect is injection site pain, which occurs because of locally recruited inflammatory mediators. Side effects may discourage individuals from receiving vaccines; therefore, reducing the duration of injection site pain can promote vaccination compliance [18].
An association between antibody response with a reactogenicity and adverse events after anti-SARS-CoV-2 vaccination has been postulated [19]. As, we are in the midst of an unprecedented pandemic, we can only speculate on the long-term effects and implications of COVID-19. Among persons with COVID-19, the development of primary fibromyalgia syndrome (FMS) is anticipated as a consequence of pandemic-associated stressors, isolation and uncertainties. Consequently, the health of these people could be profoundly and negatively affected [20] and could pave way for the presented fatigue and myalgia post-vaccination.
Only one subject had an allergic reaction. Drug hypersensitivity reactions (DHRs) were reported in 0.13% of a study from Spain on 8446 vaccinated Health Care Workers (HCWs) receiving Pfizer. Immediate DHRs related to vaccine administration are frequently associated with the inactive components or by-products of the vaccine manufacturing process, such as egg or latex or the presence of excipients such as PEG-2000 [21].
Musculoskeletal manifestations include arthralgia was shown in 14.5%, arthritis in 1.5% and LBP in 8%. The impending role of vaccines in the development of autoimmunity continues and such a link could be explained by the possible molecular mimicry between macromolecular components of the vaccine and specific human proteins and the exuberant immune response elicited by included adjuvants [11]. Arthralgia is one of the most common AEs, while isolated cases of arthritis developed after COVID-19 vaccine administration. Few had inflammatory back pain with evidence of active sacroiliitis and/or spondylitis on MRI while autoantibodies were uncommon [11]. Reactive arthritis could be induced by inactivated COVID-19 vaccination (CoronaVac, Sinovac) and patients responded well to short-term steroid therapy [22].
Since COVID-19 vaccine-related myocarditis can be fatal, it is seen in men more than women and could be overlooked. Thus, chest symptoms following the vaccine should be taken seriously and carefully monitored [23].
Pure sensitive chronic inflammatory axonal polyneuropathy has been reported in a close temporal relationship with the administration of the BNT162b2 (Pfizer®) vaccine [24]. Of the participant physicians had MS before vaccination without any AEs post-vaccination. Acute neurological deficits in the setting of recent mRNA COVID-19 vaccine administration may represent new-onset multiple sclerosis [25]. Headache was shown in 31% as presented in a prior study [26]. In this work, 16% of the subjects developed sleepliness post-vaccine. It has been speculated that the COVID-19 vaccine may be associated with and trigger hypersomnia and relentless daytime sleepiness [1].
Gastrointestinal disturbances were reported in 27% of Pakistanis receiving sinopharm vaccine and were more likely to be younger in age [27].
Enlarged thyroid and nodule developed post vaccination in 1 participant (0.5%). Aluminum is an adjuvant excipient in inactive killed COVID-19 vaccine and polyasorbate 80 (E 433) in the ChAdOx1-S recombinant vaccine [28]. Thyroid dysfunction has been reported after mRNA vaccine and inactivated vaccine [29]. Another mechanism after the COVID-19 vaccine might be an autoimmune response due to the genetic similarity of the SARS-CoV-2 spike glycoprotein with the human proteins [30].
Post-vaccine COVID-19 occurred in 15% of the subjects. Adverse reactions due to the COVID-19 vaccine were more common among participants who were previously infected with SARS CoV-2 compared to the participants previously not infected [31].
In this work, the presence of AEs post-vaccination was significantly present after 1st dose (48%) compared to 2nd dose (16.5%) or after both doses (16%). In a study from KSA, Pfizer/BioNTech vaccination was quite safe with no reported anaphylaxis or serious events. More side effects were experienced after the second dose than the first [5].
A booster dose was received by 18.5% of the subjects. Governments and health care officials should optimize the usage guidelines for COVID-19 vaccine booster doses in view of the potential waning immunity and emerging viral strains and should prioritize populations at risk in lower-income countries [32]. In a nationwide Italian study on the COVID-19 vaccine, primary vaccination efficacy was 76–92% within 6 months that decreased to 34–80% thereafter. Providing the booster doses decreased infection by 65%, hospitalizations by 69% and deaths by 97% and decreased SARS-CoV-2 infections by 39% compared to vaccine efficacy within 6 months [33].
In this work, there was a tendency that those who developed AEs were younger in age. This could be attributed to the more dynamic motion of younger subjects and higher exposure to patients in clinics. In another study, the frequency of vaccine AEs was significantly higher in the younger group with < 19 years of professional experience compared to those with ≥ 20 years [34]. As the global situation is now considering the vaccine for younger individuals and children from 5 to 11 years [35], it is mandatory that the pharmaceutical companies refine and adjust their vaccines as soon as possible with less side effects to enhance their tolerability.
Table 4 presents the post-COVID-19 infection in previous studies across the world [3, 5, 19, 31, 34, 36,37,38,39,40] compared to this work. In a recent Egyptian work, coronavirus vaccines were well-tolerated, safe, and produced an immune response against the virus in most cases. Most postvaccine AEs were mild to moderate, which indicated the building of immunity by the body for protection [41].
As this coronavirus pandemic broadens, rheumatologists having a strong background in understanding the immune system and well trained in utilizing biologics are well positioned to assist in management. Such cooperative effort should help reduce mortality during these trying times [42].
Although, this is a leading work in Egypt investigating the experience of Rheumatologists with the COVID-19 infection and vaccine in a real-world setting. A limitation of our study is the absence of a control group, e.g., physicians with other specialties or general practitioners. As the survey was announced among rheumatologist, the results cannot be generalized to other specialties. As the sampling of our study was voluntary, the possibility of a selection bias (i.e., rheumatologists with a special interest in vaccination) could not be eliminated. Information, thoughts, decisions and perceptions may be a matter of change during the ongoing pandemic. As booster doses are still starting in Egypt, and due to the time limit it was not possible to gather information on the AEs following a booster dose. Another limitation is that the analysis was based on subjects' self-reports and not on clinical diagnosis, which may introduce some information bias.
This work could at least provoke the pharmaceutical companies to revise and develop improved versions of the vaccines with the least possible side effects if humanity is destined to live with the ongoing evolving variants of COVID-19. Moreover, the additional hesitancy from receiving the vaccine calls for concerned official bodies to take action about spreading awareness about the exact safety issues related to the vaccines. Proper awareness programs and timely sharing of knowledge about the efficacy and safety of vaccines are necessary.
Taken together, our study supports the idea regarding the perception and the tolerability of COVID-19 vaccination among health care providers, could be the framework for providing high confidence in supporting their patients regarding COVID-19 vaccination. The influence of rheumatologists decision for vaccination and their experience with COVID-19 vaccine side effects on people with rheumatic diseases should be evaluated.
In conclusion, AEs of the COVID-19 vaccine were mostly mild and tolerable which makes this work in agreement with the other studies that support the broad safety of the vaccine in favor of the global benefit from mass vaccination. However, it is crucial that improvement in the pharmacokinetics of the vaccines is considered to eliminate the commonly distressing side effects. In the framework of the existing and still expanding pandemic, this work reveals the groundwork effectiveness, acceptability and AEs of the many available vaccines in Egypt that may contribute, together with other public health measures, to avoid the devastating loss of health, life, and economic and social well-being resulting from the world-wide spread of COVID-19.
Availability of data and materials
Data are available upon request from the authors.
References
Wu M, Li SX, Xue P, Zhou J, Tang X (2021) COVID-19 vaccine could trigger the relapse of secondary hypersomnia. Nat Sci Sleep. 13:2267–71
Gheita TA, Eesa NN (2019) Rheumatology in Egypt: back to the future. Rheumatol Int. 39(1):1–12
Zewude B, Habtegiorgis T, Hizkeal A, Dela T, Siraw G (2021) Perceptions and experiences of COVID-19 vaccine side-effects among healthcare workers in southern ethiopia: a cross-sectional study. PragmatObs Res. 12:131–45
Woyessa AH, Oluma A, Palanichamy T, Kebede B, Abdissa E, Labata BG et al (2021) Predictors of health-care workers’ unwillingness to continue working during the peak of COVID-19 in Western Ethiopia: an extended parallel-process model study. Risk Manag Health Policy. 14:1165–73
Mohammed RA, Garout RM, Wahid S, Ayub F, FirasZinAlddin LM et al (2021) A survey on the side effects of Pfizer/BioNTech COVID-19 vaccine among vaccinated adults in Saudi Arabia. Cureus. 13(11):e19222
Butler-Manuel W, Rana UI, Zafar M, Gadi A, Kiani A (2022) Post COVID-19 vaccine related cerebral venous sinus thrombosis and thrombocytopenia. Cureus. 14(1):e20932
Hammam N, Tharwat S, Shereef RRE, Elsaman AM, Khalil NM, Egyptian College of Rheumatology (ECR) COVID-19 Study Group et al (2021) Rheumatology university faculty opinion on coronavirus disease-19 (COVID-19) vaccines: the vaXurvey study from Egypt. Rheumatol Int. 41(9):1607–16
Whitaker HJ, Tsang RS, Byford R, Andrews NJ, Sherlock J, Pillai PS et al (2022) Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response among individuals in clinical risk groups. J Infect. S0163–4453(21):00664–2
Sen P, Gupta L, Lilleker JB, Aggarwal V, Kardes S, COVAD Study Group (2022) COVID-19 vaccination in autoimmune disease (COVAD) survey protocol. Rheumatol Int. 42(1):23–29
Eysenbach G (2004) Improving the quality of web surveys: the checklist for reporting results of internet E-surveys (CHERRIES). J Med Internet Res. 6(3):e132
Ursini F, Ruscitti P, Raimondo V, De Angelis R, Cacciapaglia F, Pigatto E et al (2021) Spectrum of short-term inflammatory musculoskeletal manifestations after COVID-19 vaccine administration: a report of 66 cases. Ann Rheum Dis. 81(3):440–441
Gheita TA, Salem MN, Eesa NN, Khalil NM, Gamal NM, ECR COVID19-Study Group (2020) Rheumatologists’ practice during the Coronavirus disease 2019 (COVID-19) pandemic: a survey in Egypt. Rheumatol Int. 40(10):1599–611
Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE et al (2021) Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 326:1390–9
Mutombo PN, Fallah MP, Munodawafa D, Kabel A, Houeto D, Goronga T et al (2021) COVID-19 vaccine hesitancy in Africa: a call to action. Lancet Glob Health. 10(3):e320–e321
Gong IY, Vijenthira A, Betschel SD, Hicks LK, Cheung MC (2022) COVID-19 vaccine response in patients with hematologic malignancy: a systematic review and meta-analysis. Am J Hematol. https://doi.org/10.1002/ajh.26459
Molica S, Giannarelli D, Lentini M, Zappala D, Mannella A, Loiacono D et al (2021) Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia: a serologic and cellular study. Chemotherapy. https://doi.org/10.1159/000521229 (Epub ahead of print)
Ontario Agency for Health Protection and Promotion (Public Health Ontario) (2021) Weekly summary: adverseevents following immunization (AEFIs) for COVID-19 in Ontario: December 13, 2020 to January 9, 2022. Queen’s Printer for Ontario, Toronto
Marshall S, Winter S, Capobianco JD (2022) Lymphatic osteopathic manipulative treatment reduces duration of deltoid soreness after Pfizer/BioNTech COVID-19 vaccine. J Osteopath Med. 122(3):153–157
Lim SH, Choi SH, Kim B, Kim JY, Ji YS, Kim SH et al (2021) Serum antibody response comparison and adverse reaction analysis in healthcare workers vaccinated with the BNT162b2 or ChAdOx1 COVID-19 vaccine. Vaccines (Basel). 9(12):1379
Gheita TA, Fathi HM, ElAdle SS, Eesa NN, Hammam NH (2021) Coronavirus disease 2019 (COVID-19) an emerging trigger for primary fibromyalgia syndrome: a tale of three cases post-COVID-19. Int J Clin Rheumatol. 16(4):129–35
LoliAusejo D, de González Abreu JM, Fiandor A, Cabañas R, Domínguez-Ortega F, Caballero ML et al (2021) Allergic reactions after administration of Pfizer-BioNTech COVID-19 vaccine to health care workers at a tertiary hospital. J InvestigAllergol Clin Immunol. 31(6):507–8
Türk SM, Öztürk Z, Karataş D, Gönüllü E (2021) Inactivated COVID-19 vaccine can induce rective polyarthritis in older patients: report of 2 cases. Georgian Med News. 319:100–102
Akase B, Hayashi K, Hisada T, Tsuchiya T, Masaki N, Nagata M (2021) Chest pain with new abnormal electrocardiogram development after injection of COVID-19 vaccine manufactured by moderna. Intern Med. https://doi.org/10.2169/internalmedicine.8711-21
Luca A, Squillaci R, Terravecchia C, Contrafatto F, Reggio E, Nicoletti A et al (2021) Pure sensitive chronic inflammatory axonal polyneuropathy following Pfizer COVID-19 vaccine. Neurol Sci. 43(2):1431–1433
Toljan K, Amin M, Kunchok A, Ontaneda D (2022) New diagnosis of multiple sclerosis in the setting of mRNA COVID-19 vaccine exposure. J Neuroimmunol. 362:577785 (Epub ahead of print)
Ekizoglu E, Gezegen H, YalınayDikmen P, Orhan EK, Ertaş M, Baykan B (2022) The characteristics of COVID-19 vaccine-related headache: clues gathered from the healthcare personnel in the pandemic. Cephalalgia. 42(4–5):366–375
Abbas S, Abbas B, Amir S, Wajahat M (2021) Evaluation of adverse effects with COVID-19 vaccination in Pakistan. Pak J Med Sci. 37(7):1959–64
Liang Z, Zhu H, Wang X, Jing B, Li Z, Xia X et al (2020) Adjuvants for coronavirus vaccines. Front Immunol 11:589833
Lee KA, Kim YJ, Jin HI (2021) Thyrotoxicosis after COVID-19 vaccination: seven case reports and a literature review. Endocrine 74:470–72
Chen Y, Tian Y, Li Z, Zhu T, Wei J, Lei J (2021) Potential interaction between SARS-CoV-2 and thyroid: a review. Endocrinology 162(3):bqab004
Azimi M, Dehzad WM, Atiq MA, Bahain B, Asady A (2021) Adverse effects of the COVID-19 vaccine reported by lecturers and staff of Kabul University of Medical Sciences, Kabul Afghanistan. Infect Drug Resist. 14:4077–83
Shekhar R, Garg I, Pal S, Kottewar S, Sheikh AB (2021) COVID-19 Vaccine Booster: to boost or not to boost. Infect Dis Rep. 13(4):924–9
Mattiuzzi C, Lippi G (2022) Primary COVID-19 vaccine cycle and booster doses efficacy: analysis of Italian nationwide vaccination campaign. Eur J Public Health. 32(2):328–330
Tosun S, OzkanOzdemir H, Erdogan E, Akcay S, Aysin M, Eskut N et al (2021) Adverse events report of inactivated COVID-19 vaccine from 4040 healthcare workers. Postgrad Med. 134:104–110
Dobrina R, Bicego L (2022) COVID-19 vaccine campaign has now opened up for children aged 5–11. How are kids going to live it? J Pediatr Nurs. S0882–5963(21):00388–2
Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE et al (2021) Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw Open. 4(12):e2140364
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S et al (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 383(27):2603–15
Borroni E, Consonni D, Cugno M, Lombardi A, Mangioni D, Bono P et al (2021) Side effects among healthcare workers from a large Milan university hospital after second dose of BNT162b2 mRNA COVID-19 vaccine. Med Lav. 112(6):477–85
Khan MK, Ferdous J, Akhter S, Esha AM, Islam M (2022) Tracking side effects of the COVID-19 vaccine in Mymensingh district of Bangladesh. Mymensingh Med J. 31(1):1–9
Babamahmoodi F, Saeedi M, Alizadeh-Navaei R, Hedayatizadeh-Omran A, Mousavi SA, Ovaise G et al (2021) Side effects and Immunogenicity following administration of the Sputnik V COVID-19 vaccine in health care workers in Iran. Sci Rep. 11(1):21464
Elgendy MO, El-Gendy AO, Mahmoud S, Mohammed TY, Abdelrahim MEA, Sayed AM (2022) Side effects and efficacy of COVID-19 vaccines among the Egyptian population. Vaccines. 10(1):109
Gheita TA, Kenawy SA (2020) Egypt’s groundwork blessing during the COVID-19 pandemic curse: Rheumatologic experience. Eur J Rheumatol. 7(Suppl 2):S134–S136
Acknowledgements
The authors thank all respondents for participating in the survey. Appreciation is due to the members of the Egyptian College of Rheumatology for their invaluable contribution in the dissemination of this survey which made the data collection possible.
Funding
No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work describes in this manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
GT and FHM: conceived and designed the study. Data collection and revision were conducted by GT, FHM and HN. GT: performed analysis, and along with FH and HN: draft the manuscript. All authors reviewed and approved the final manuscript. All co-authors take full responsibility for the integrity and accuracy of all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Research involving human participants
Rheumatologists from 15 universities and institutions conducted an e-survey to the colleagues in various governorates that are willing to participate. Moreover, the survey was shared on relevant social media platforms on WhatsApp and Facebook.
Informed consent
consent of participation was granted and fulfilled by those, who actually shared in this e-survey.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fathi, H.M., Gazzar, I.I.E., Elazeem, M.I.A. et al. Rheumatologists’ knowledge and perception of COVID-19 and related vaccines: the vaXurvey2 online survey. Rheumatol Int 42, 989–998 (2022). https://doi.org/10.1007/s00296-022-05130-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05130-3