Abstract
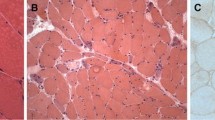
Juvenile dermatomyositis (JDM) is an inflammatory myopathy which causes severe morbidity and high mortality if untreated. In this study, we aimed to define the T-helper cell profile in the muscle biopsies of JDM patients. Muscle biopsies of twenty-six patients (50% female) were included in the study. Immunohistochemical expression of CD3, CD20, CD138, CD68, IL-17, Foxp3, IFN-ɣ, IFN-alpha and IL-4 was studied and muscle biopsies were scored using the JDM muscle biopsy scoring tool. Inflammatory cells were in small clusters in perimysium and perivascular area or scattered throughout the endomysium in most biopsies; however in 2 biopsies, lymphoid follicle-like big clusters were observed, and in one, there was a very dense and diffuse inflammatory infiltration nearly destroying all the muscle architecture. Seventy-three per cent of the biopsies had T cells, 88% had B cells, 57% had plasma cells, and all had macrophages. As for T-helper cell subtypes, 80% of the biopsies were Th1 positive, 92% Th17 positive and 30% Treg positive. No IL-4 positive inflammatory cell was detected, and only 2 biopsies showed IFN-alpha positivity. The mean JDM biopsy score was 17.6, meaning moderate to severe muscular involvement. Visual analogue score of the pathologist was strongly correlated with histopathological features. B cells, macrophages, plasma cells and T cells constitute the inflammatory milieu of the JDM muscle biopsies. As for T cells, JDM is a disease mainly related with Th1 and Th17 T-helper cell subtypes and to some extend Treg. Th2 cells are not involved in the pathogenesis.





Similar content being viewed by others
References
Wedderburn LR, Rider LG (2009) Juvenile dermatomyositis: new developments in pathogenesis, assessment and treatment. Best Pract Res Clin Rheumatol 23(5):665–678
Unverricht H (1891) Dermatomyositis acuta. DTsch Med Wochenschr 17:41–44
Bohan A, Peter JB (1975a) Polymyositis and dermatomyositis (second of two parts). N Engl J Med 292(8):403–407
Bohan A, Peter JB (1975b) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292(7):344–347
Robinson AB, Reed AM (2011) Clinical features, pathogenesis and treatment of juvenile and adult dermatomyositis. Nat Rev Rheumatol 7(11):664–675
Li CK et al (2004) MHC Class I overexpression on muscles in early juvenile dermatomyositis. J Rheumatol 31(3):605–609
Chevrel G et al (2003) Interleukin-17 increases the effects of IL-1 beta on muscle cells: arguments for the role of T cells in the pathogenesis of myositis. J Neuroimmunol 137(1–2):125–133
de Padilla CML et al (2007) Plasmacytoid dendritic cells in inflamed muscle of patients with juvenile dermatomyositis. Arthritis Rheum 56(5):1658–1668
Baechler EC, Bilgic H, Reed AM (2011) Type I interferon pathway in adult and juvenile dermatomyositis. Arthritis Res Ther 13(6):249
Kao L, Chung L, Fiorentino DF (2011) Pathogenesis of dermatomyositis: role of cytokines and interferon. Curr Rheumatol Rep 13(3):225–232
Salomonsson S, Lundberg IE (2006) Cytokines in idiopathic inflammatory myopathies. Autoimmunity 39(3):177–190
Lundberg I et al (1997) Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum 40(5):865–874
Soponkanaporn S et al (2019) Expression of myxovirus-resistance protein A: a possible marker of muscle disease activity and autoantibody specificities in juvenile dermatomyositis. Neuropathol Appl Neurobiol 45(4):410–420
Jager A, Kuchroo VK (2010) Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol 72(3):173–184
Palmer MT, Weaver CT (2010) Autoimmunity: increasing suspects in the CD4+ T cell lineup. Nat Immunol 11(1):36–40
Steinman L (2010) Mixed results with modulation of TH-17 cells in human autoimmune diseases. Nat Immunol 11(1):41–44
Wedderburn LR et al (2007) International consensus on a proposed score system for muscle biopsy evaluation in patients with juvenile dermatomyositis: a tool for potential use in clinical trials. Arthritis Rheum 57(7):1192–1201
Varsani H et al (2015) Validation of a score tool for measurement of histological severity in juvenile dermatomyositis and association with clinical severity of disease. Ann Rheum Dis 74(1):204–210
Deakin CT et al (2016) Muscle biopsy findings in combination with myositis-specific autoantibodies aid prediction of outcomes in juvenile dermatomyositis. Arthritis Rheumatol 68(11):2806–2816
Yasin SA et al (2019) Histological heterogeneity in a large clinical cohort of juvenile idiopathic inflammatory myopathy: analysis by myositis autoantibody and pathological features. Neuropathol Appl Neurobiol 45(5):495–512
Tansley SL et al (2014) Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther 16(4):R138
Lopez De Padilla CM et al (2009) Extranodal lymphoid microstructures in inflamed muscle and disease severity of new-onset juvenile dermatomyositis. Arthritis Rheum 60(4):1160–1172
Oddis CV et al (2013) Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum 65(2):314–324
Bader-Meunier B et al (2011) Safety and efficacy of rituximab in severe juvenile dermatomyositis: results from 9 patients from the French Autoimmunity and Rituximab registry. J Rheumatol 38(7):1436–1440
Bellutti Enders F et al (2017) Consensus-based recommendations for the management of juvenile dermatomyositis. Ann Rheum Dis 76(2):329–340
Aggarwal R et al (2014) Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol 66(3):740–749
Hamaguchi Y et al (2011) Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol 147(4):391–398
Shah M et al (2013) The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 92(1):25–41
Stone KB et al (2007) Anti-Jo-1 antibody levels correlate with disease activity in idiopathic inflammatory myopathy. Arthritis Rheum 56(9):3125–3131
Bilgic H et al (2009) Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum 60(11):3436–3446
Baechler EC et al (2007) An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med 13(1–2):59–68
Salajegheh M et al (2010) Interferon-stimulated gene 15 (ISG15) conjugates proteins in dermatomyositis muscle with perifascicular atrophy. Ann Neurol 67(1):53–63
Chinoy H et al (2007) Interferon-gamma and interleukin-4 gene polymorphisms in Caucasian idiopathic inflammatory myopathy patients in UK. Ann Rheum Dis 66(7):970–973
De Rossi M et al (2000) Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation. Int Immunol 12(9):1329–1335
Jager A et al (2009) Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol 183(11):7169–7177
Bettelli E et al (2008) Induction and effector functions of T(H)17 cells. Nature 453(7198):1051–1057
Crowson CS et al (2019) Interferon chemokine score and other cytokine measures track with changes in disease activity in patients with juvenile and adult dermatomyositis. ACR Open Rheumatol 1(2):83–89
Coates LC et al (2016) Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 68(5):1060–1071
Baeten D et al (2013) Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 382(9906):1705–1713
Bettelli E et al (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090):235–238
Vercoulen Y et al (2014) Increased presence of FOXP3+ regulatory T cells in inflamed muscle of patients with active juvenile dermatomyositis compared to peripheral blood. PLoS ONE 9(8):e105353
Fujiyama T et al (2014) Preferential infiltration of interleukin-4-producing CXCR4+ T cells in the lesional muscle but not skin of patients with dermatomyositis. Clin Exp Immunol 177(1):110–120
Horsley V et al (2003) IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113(4):483–494
Acknowledgements
This project was supported by Hacettepe University Scientific Research Projects Coordination Unit with the project number 014 D04 101 012-575.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception or design of this study, or to the generation, analysis and/or interpretation of data, and agree to be accountable for the integrity of the work herein. All authors reviewed the manuscript and approved the submitted version. No person who fulfils the criteria for authorship has been excluded as an author.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
This study was approved by Hacettepe University Ethical Committee (GO 13/582-30).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sag, E., Kale, G., Haliloglu, G. et al. Inflammatory milieu of muscle biopsies in juvenile dermatomyositis. Rheumatol Int 41, 77–85 (2021). https://doi.org/10.1007/s00296-020-04735-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04735-w