Abstract
Accurate measurement of sedentary time and physical activity (PA) is essential to establish their relationships with rheumatoid arthritis (RA) outcomes. Study objectives were to: (1) validate the GT3X+ and activPAL3μ™, and develop RA-specific accelerometer (count-based) cut-points for measuring sedentary time, light-intensity PA and moderate-intensity PA (laboratory-validation); (2) determine the accuracy of the RA-specific (vs. non-RA) cut-points, for estimating free-living sedentary time in RA (field-validation). Laboratory-validation: RA patients (n = 22) were fitted with a GT3X+, activPAL3μ™ and indirect calorimeter. Whilst being video-recorded, participants undertook 11 activities, comprising sedentary, light-intensity and moderate-intensity behaviours. Criterion standards for devices were indirect calorimetry (GT3X+) and direct observation (activPAL3μ™). Field-validation: RA patients (n = 100) wore a GT3X+ and activPAL3μ™ for 7 days. The criterion standard for sedentary time cut-points (RA-specific vs. non-RA) was the activPAL3μ™. Results of the laboratory-validation: GT3X—receiver operating characteristic curves generated RA-specific cut-points (counts/min) for: sedentary time = ≤ 244; light-intensity PA = 245–2501; moderate-intensity PA ≥ 2502 (all sensitivity ≥ 0.87 and 1-specificity ≤ 0.11). ActivPAL3μ™—Bland–Altman 95% limits of agreement (lower–upper [min]) were: sedentary = (− 0.1 to 0.2); standing = (− 0.7 to 1.1); stepping = (− 1.2 to 0.6). Results of the field-validation: compared to the activPAL3μ™, Bland–Altman 95% limits of agreement (lower–upper) for sedentary time (min/day) estimated by the RA-specific cut-point = (− 42.6 to 318.0) vs. the non-RA cut-point = (− 19.6 to 432.0). In conclusion, the activPAL3μ™ accurately quantifies sedentary, standing and stepping time in RA. The RA-specific cut-points offer a validated measure of sedentary time, light-intensity PA and moderate-intensity PA in these patients, and demonstrated superior accuracy for estimating free-living sedentary time, compared to non-RA cut-points.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research evidence supports the benefits of physical activity (PA) for improving health-related outcomes among people with rheumatoid arthritis (RA) [1]. More recently, studies also suggest sedentary behaviour (waking behaviour ≤ 1.5 metabolic equivalents [METs], whilst in a sitting, reclining or lying posture) [2, 3] is adversely associated with RA outcomes [4]. However, most evidence regarding the role of sedentary time and PA in RA is based on studies employing self-report methods to quantify engagement in these behaviours [4, 5].
Device-based assessments of sedentary time and PA offer a more objective measure of behaviour, and have demonstrated higher validity and reliability relative to self-report instruments [6,7,8]. Consequently, devices are being more readily used to measure sedentary time and PA in different populations, including in RA [4, 9]. Currently, hip-worn accelerometers (e.g., ActiGraph [Florida, USA]) are the most commonly employed device in RA studies to estimate the frequency, intensity and duration of free-living behaviour. The accelerometer records and stores raw acceleration data (g), which is subsequently processed to provide estimates of sedentary behaviour and PA. Currently, several processing methods can be applied to raw accelerometer data, with the dominant approach being the use of thresholds or ‘cut-points’ that classify behaviour as sedentary, light-intensity PA (LPA), moderate-intensity PA (MPA) or vigorous-intensity PA. There is an absence of a consensus on the ‘best’ method, with this decision dependent on the research question, study resources and research team expertise [10].
A popular and widely accessible data processing method generates sedentary time and PA estimates by applying cut-points to accelerometer activity counts (‘count-based cut-points’) that have been derived from raw accelerometer data using the device manufacturer’s proprietary software. These count-based cut-points are commonly employed, largely due to intuitive and easy-to-use software platforms that facilitate straightforward processing and analysis of complex raw accelerometer data, thus, making the application of accelerometry accessible to researchers from a wide range of disciplines (e.g., clinical/medicine, exercise science, behavioural science). However, whilst the advantages of accelerometry (and specifically, count-based cut-points) to measure sedentary time and PA are being increasingly recognised by RA researchers, several limitations exist regarding their application in this patient group.
First, few accelerometers have been specifically validated for measurement of sedentary time and PA in RA (e.g., against indirect calorimetry). Consequently, existing RA studies employing accelerometers have largely employed count-based cut-points developed in validation studies of healthy participants [11, 12] to quantify sedentary time and PA in RA. However, as RA patients differ markedly to people without RA in terms of physiology, physical function and associated activity patterns (e.g., RA patients demand a relatively higher basal metabolic rate compared to the general population [13]), such sedentary time and PA estimates should be interpreted with caution.
Second, most existing count-based cut-points are uniaxial, generating sedentary time and PA estimates using data captured by a single axis of movement. Technological advancements are such that triaxial accelerometers are now common place, and can capture data across three axes (Y, X and Z) to provide a more valid assessment of behaviour [14]. Thus, given the increasing popularity of applying count-based cut-points to examine sedentary time and PA in RA studies, there is a critical need to develop RA-specific triaxial accelerometer count-based cut-points to provide a valid and accessible accelerometer data processing method for RA researchers.
Still, a key limitation of accelerometers is their inability to distinguish sitting (sedentary behaviour) from standing without movement (LPA). Specifically, accelerometers work on the basis that all movements registered below a ‘sedentary time cut-point’ are by default, classed as sedentary [15]. However, low-movement behaviours may occur in a sitting or standing posture, but both may record accelerations that register below the ‘sedentary time cut-point’. Thus, accelerometers may lead to an overestimation of sedentary time by misclassifying low-movement standing behaviours as sitting (sedentary). The activPAL™ (PAL Technologies, Glasgow, UK) addresses this limitation, and is able to accurately classify behaviours as sitting/lying (sedentary), standing or stepping. This device is currently considered the gold standard for measurement of free-living sedentary time [6]. Thus, the activPAL™ primarily offers a measure of sedentary behaviour, rather than frequency, intensity and duration of PA. Consequently, few RA studies have employed the activPAL™, with extant research employing this device focusing specifically on the role of sedentary behaviour [16].
Considering exponential growth in research centred on the role of sedentary behaviour and PA for improving RA disease outcomes, it is critical that device-based measures are properly validated for use in this population. Therefore, the overarching aim of the current study was to validate the commonly employed ActiGraph GT3X+ and the activPAL3μ™, for measurement of sedentary time and PA in RA. In a laboratory-validation (objective 1), this study aimed to: (a) validate the GT3X+ against indirect calorimetry to generate RA-specific triaxial (vector magnitude [VM]) accelerometer count-based cut-points for sedentary time, LPA and MPA; (b) validate the activPAL3μ™ against direct observation for measurement of sedentary, standing and stepping time. Then, using these data, conduct a field-validation (objective 2) to compare the validity of the new RA-specific triaxial sedentary time count-based cut-point vs. a widely used non-RA uniaxial sedentary time count-based cut-point (< 100 counts/min) [11, 12] for measurement of free-living sedentary time in RA, against the gold standard (activPAL3μ™).
Materials and methods
Participants and recruitment
Participants were recruited from outpatient clinics at Russells Hall Hospital (Dudley Group NHS Foundation Trust). The only requirements for inclusion in this study were a clinical diagnosis of RA according to the American College of Rheumatology/European League Against Rheumatism classification criteria [17], and aged ≥ 18 years. For objective 1, patients were required to ambulate independently. For objective 2, patients were eligible if they could ambulate independently, or with an assistive device. Participants were excluded from objectives 1 and 2 if they were pregnant. Eligibility criteria were intentionally broad in order that the GT3X+ and activPAL3μ™ were validated in a more diverse population of people living with RA (e.g., males and females; low, moderate and high disease activity). All participants provided written informed consent. This study was approved by the local National Health Service Research Ethics Committee (16/WM/0371).
Protocol
The protocol for this study has been previously published [18], but methods and analytical approaches are briefly described herein.
Objective 1 (laboratory-validation)
Participants (n = 22) reported to the laboratory following a 12-h fast, and having refrained from exercise for 48 h. Upon arrival, participants completed physical assessments (e.g., height, weight, body-mass index), and underwent routine clinical evaluations to determine their disease activity (disease activity score-28 [19]) and level of functional disability (health assessment questionnaire [20]). Participants were then fitted with the GT3X+, activPAL3μ™, heart rate monitor (Polar Electro Oy Ltd., Kempele, Finland) and Cortex Metalyzer® 3B (indirect calorimeter [Cortex Biophysik, Leipzig, Germany]) for the duration of the laboratory-validation. For direct observation of behaviour, a video camera was set up overlooking the laboratory. All equipment was time-synchronised.
Participants undertook 11 activities (6 standardised activities and 5 activities of daily living [ADLs]). Activities required between 1.3 and 3.5 METs (ranging from sedentary behaviour to MPA) and were 6-min in duration [21]. Five-min rest periods separated the ADLs, to allow heart rate and VO2 to return to resting levels [14, 21, 22].
Objective 2 (field-validation)
Participants (n = 104) attended the laboratory to complete physical assessments and routine clinical evaluations, as per objective 1. Participants were asked to wear the GT3X+ and activPAL3μ™ for 7 days to assess free-living sedentary time and PA [23]. The GT3X+ was worn during all waking hours, removing for water-based activities. The activPAL3μ™ was worn continuously for 24 h/day.
Measures
Devices
The GT3X+ is a triaxial accelerometer that records accelerations on three axes (vertical [Y], horizontal right-left [X] and horizontal front-back [Z]), over researcher-defined time periods (epochs). These data are used to compute VM [VM = √(axisY2 + axisX2 + axisZ2)], which is used to quantify sedentary time and PA. The GT3X+ accelerometers were set to sample movement in 1-s epochs at a rate of 30 Hz. For objectives 1 and 2, participants wore the GT3X+ attached to an elastic belt on their right hip [12, 22, 24, 25].
The activPAL3μ™ is an accelerometer with inclinometer function, that measures free-living behaviour over consecutive 24-h periods. For objectives 1 and 2, the activPAL3μ™ was worn in a mid-anterior position on the right thigh, attached with a waterproof adhesive dressing [26].
Criterion standards
Indirect calorimetry was the criterion standard for validating the GT3X+. The Cortex Metalyzer® 3B uses a breath-by-breath system to directly measure an individual’s concentration of inspired oxygen (O2) and expired carbon dioxide (CO2) to calculate VO2 (ml/kg/min) and METs, using MetaSoft® (Cortex Biophysik). Direct observation (via video camera) was the criterion standard for validating the activPAL3μ™.
Following laboratory-validation of the activPAL3μ™, this device was employed as the criterion standard for assessing the accuracy of the RA-specific triaxial vs. the non-RA uniaxial sedentary time count-based cut-point. This decision was based on prior studies demonstrating high validity of the activPAL3μ™ for estimating free-living sedentary time in RA, recognising this device as the current gold standard for measurement of free-living sedentary time [6].
Data reduction and statistical analysis
Objective 1 (laboratory-validation)
GT3X+ and indirect calorimetry
The manufacturer’s software (Actilife [ActiGraph]) was used to download time-stamped GT3X+ data in the format of triaxial (VM) activity counts. Data were downloaded in counts/s, and converted to counts/min for analysis.
Metasoft® was used to download and export breath-by-breath VO2 data from the Cortex Metalyzer® 3B. In Microsoft Excel, second-by-second VO2 data were averaged across each minute to compute average VO2 (ml/kg/min) per minute of activity. These data were graphed to identify when steady-state VO2 was achieved within each activity (steady-state = variation within ± 0.5 ml/kg/min). Graphed data indicated steady-state occurred in min 4–6 of each activity (the final 2 min of the 11 activities). VO2 (ml/kg/min) and GT3X+ (counts/min) data were therefore averaged across min 4–6 of each laboratory testing component, to provide steady-state VO2 and GT3X+ data for each activity. These data were exported into SPSS (Chicago, USA, v.24) for statistical analysis. Where participants did not reach steady-state VO2 during an activity, their data recorded for that particular activity were excluded.
Statistical analysis
Average (steady-state) VO2 data were converted into METs (1 MET = 3.5 ml/kg/min) and then classified as sedentary (≤ 1.5 METs), LPA (1.6–2.9 METs) or MPA (≥ 3 METs). Using these classifications, data were recoded to create binary variables for use in receiver operating characteristic (ROC) curve analysis, to define RA-specific triaxial (VM) accelerometer count-based cut-points for sedentary time, LPA and MPA. Specifically, data were recoded as sedentary/not sedentary or MPA/not MPA using binary indicators (1/0). ROC curves identified the VM activity count maximising sensitivity (Y-axis) and specificity (X-axis) for correctly classifying behaviour as sedentary or MPA. Area under the curve (AUC) values were also calculated (AUC criteria: 0.90–1.00 = excellent; 0.80–0.89 = good; 0.70–0.79 = fair; 0.60–0.69 = poor; < 0.60 = failure).
ActivPAL3μ™ and direct observation
PAL Connect (PAL Technologies) was used to download and export activPAL3μ™ time-stamped data to Microsoft Excel. Outputs displayed sedentary, standing and stepping time, and number of steps and sit-stand transitions, for consecutive 15-s periods for the duration of the laboratory-validation. For direct observation, the researcher observed all video camera recordings, recording engagement in sitting/lying (sedentary), standing or stepping, as well as counting steps and sit-stand transitions, every 15 s for each activity.
Statistical analysis
Means (M) and standard deviations (SD) were calculated for activPAL3μ™-assessed and directly observed sedentary, standing and stepping time (min), and steps and sit-stand transitions (number). Bland–Altman analysis calculated 95% limits of agreement (LOA [lower to upper]) between activPAL3μ™-assessed vs. directly observed behaviours, using the M and SD of the differences (min) between the two measures [M ± (SD × 1.96)] [27, 28]. Finally, percentage accuracy for activPAL3μ™-assessment vs. direct observation of behaviours was computed [% accuracy = (activPAL3μ™ value/direct observation value) × 100].
Objective 2 (field-validation)
Actilife was used to download 7-day GT3X+ data (1-s epochs) and check non-wear (criteria = ≥ 60 min of consecutive zero counts, spike tolerance of 2 min) [9, 12]. All non-wear periods identified were excluded from each participant’s data file. After removing non-wear periods, participants’ 7-day GT3X+ data were retained for inclusion in statistical analysis where GT3X+ accelerometers were worn for ≥ 10 h/day on ≥ 4 days (including ≥ 1 weekend day) [9, 12]. The RA-specific triaxial (VM) accelerometer count-based cut-point (developed in objective 1) and non-RA uniaxial (Y-axis) accelerometer count-based cut-point (< 100 counts/min) [11, 12], were then applied to 7-day GT3X+ data to derive estimates of free-living sedentary time (min/day).
For the activPAL3μ™, PAL Connect was used to download and export daily movement data (15-s epochs) that corresponded to valid days measured via the GT3X+ . Sleep time was manually removed from activPAL3μ™ data using wear-time logbooks and sleep-periods identified from GT3X+ data analysis. Estimates of free-living activPAL3μ™-assessed sedentary time (min/day) were calculated using PAL Connect proprietary algorithms.
Statistical analysis For objective 2, Bland–Altman analysis was used to calculate 95% LOA (lower to upper) between GT3X+- and activPAL3μ™-assessed free-living sedentary time, for both RA-specific and non-RA count-based cut-points. LOA were determined using the M and SD of the differences (min/day) between estimates of GT3X +- and activPAL3μ™-assessed sedentary time [M ± (SD × 1.96)].
Results
Objective 1 (laboratory-validation)
Twenty-two patients (86% female, n = 19) participated in the laboratory protocol (Table 1). GT3X+ and indirect calorimetry: Table 2 reports the M (SD) for GT3X+ activity counts and METs during steady-state VO2. Activity intensities (METs) reflecting sedentary, LPA and MPA were achieved as intended. Table 3 reports results of ROC curve analysis and the RA-specific triaxial (VM) accelerometer count-based cut-points maximising sensitivity and specificity. The AUC demonstrated ‘excellent’ fit for RA-specific sedentary time (AUC = 1.00) and MPA (AUC = 0.94) count-based cut-points. ActivPAL3μ™ and direct observation: Table 4 reports the M (SD) for activPAL3μ™-assessed and directly observed behaviours during the laboratory testing procedure. Compared to direct observation, the activPAL3μ™ accurately classified sedentary, standing and stepping time, and step number, > 98% of the time. For number of sit-stand transitions, classification accuracy was 72%.
Mean differences for activPAL3μ™-assessed vs. directly observed behaviours were computed (M [SD]): sedentary time = 0.1 (0.1) min; standing = 0.2 (0.5) min; stepping = − 0.3 (0.5) min; steps = − 30 (44); sit-stand transitions = − 2 (1). Bland–Altman analysis (Fig. 1) demonstrated narrow 95% LOA (lower to upper) for sedentary (− 0.1 to 0.2), standing (− 0.7 to 1.1) and stepping (− 1.2 to 0.6) time (min). For number of steps, 95% LOA were wider (− 116 to 57). As only M (SD) = 5 (1) and M (SD) = 7 (0) sit-stand transitions were recorded by the activPAL3μ™ and direct observation, respectively, Bland–Altman plots could not be produced for this outcome.
Objective 1: Bland–Altman plots showing agreement (mean difference and 95% limits of agreement [LOA]) for time spent sedentary (a), standing (b), and stepping (c), as well as number of steps (d), between the activPAL3μ™ vs. direct observation. Note: Straight full line represents mean difference and the straight dotted line represents lower and upper LOA (95%)
Objective 2 (field-validation)
A total of n = 100 participants (96% [71% female, n = 71]) provided valid 7-day GT3X+ and corresponding activPAL3μ™ data (Table 1). GT3X+-derived sedentary time estimates (M [SD]) were: RA-specific count-based cut-point = 686.1 (72.4) min/day vs. non-RA count-based cut-point = 754.7 (62.5) min/day.
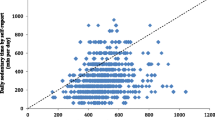
For the RA-specific count-based cut-point (≤ 244 counts/min) vs. the activPAL3μ™, Bland–Altman analysis (Fig. 2) revealed a mean difference of 137.7 (SD = 92.0), with 95% LOA (lower to upper) = (− 42.6 to 318.0), for sedentary time (min/day). Most data points were positioned above zero and followed a downward trend, whereby a lower mean difference between measures was observed at higher levels of sedentary time.
Objective 2: Bland–Altman plots showing agreement (mean difference and limits of agreement [LOA]) between GT3X+-assessed vs. activPAL3μ™-assessed sedentary time. Accelerometer count-based cut-points applied were: RA-specific (VM) count-based cut-points [≤ 244 count/min, derived from objective 1 of this study (a)], and non-RA (Y-axis) count-based cut-points [< 100 counts/min (b)]. Note: Straight full line represents mean difference and the straight dotted line represents lower and upper LOA (95%)
Compared to the RA-specific triaxial count-based cut-point, the non-RA uniaxial count-based cut-point demonstrated a greater mean difference (206.2 [SD = 115.2]) and wider 95% LOA (lower to upper) = (− 19.6 to 432.0) vs. the activPAL3μ™ for sedentary time (min/day). Bland–Altman analysis for the non-RA count-based cut-point revealed most data points were scattered above zero, and a downward trend was observed (lower mean difference between measures at higher levels of sedentary time).
Discussion
The current study validated the ActiGraph GT3X+ and activPAL3μ™—two devices commonly used in sedentary behaviour and PA research—for measurement of sedentary time and PA in people living with RA. Whilst there are several options for processing raw accelerometer data to quantify sedentary time and PA in healthy populations, count-based cut-points offer an accessible means of accelerometer data processing for researchers and health professionals working in rheumatology. To date, RA studies employing accelerometers have largely relied on the application of non-RA count-based cut-points to quantify free-living sedentary time and PA in this population [29, 30], which are limited in their validity when we consider the unique physiology and associated movement patterns of people living with RA [21, 22, 24]. Thus, there exists a critical need for the development of RA-specific count-based cut-points, which can be easily and consistently employed across RA studies.
In response, this is the first study to calibrate the commonly employed GT3X+ and define RA-specific triaxial accelerometer count-based cut-points, for valid measurement of sedentary time, LPA and MPA in RA. Our RA-specific count-based cut-points were derived according to energy requirements of behaviour among people with RA, and demonstrated high sensitivity and specificity for classification of sedentary time, LPA and MPA. Thus, the application of our novel RA-specific triaxial count-based cut-points are likely to provide more valid assessments of sedentary time and PA in RA, relative to employing non-RA uniaxial count-based cut-points developed in validation studies of healthy adults. We therefore recommend using the RA-specific count-based cut-points proposed herein, in future RA research.
This study also assessed the accuracy of the activPAL3μ™ for measurement of sedentary, standing and stepping time in RA. Only one study has examined the ability of the activPAL™ to validly assess posture in RA [31]. Larkin et al. [31] employed regression analysis and observed strong associations between activPAL™-assessed sedentary, standing and stepping time with directly observed behaviour. However, it would be surprising to find a non-significant relationship between two methods designed to measure the same variables [27, 28]. Thus, we employed Bland–Altman analysis to determine agreement between activPAL3μ™-assessed vs. directly observed behaviours [27, 32], and reported high classification accuracy (> 98%) between the two measures for all behaviours, in our sample of RA patients. This is in line with past research in non-RA populations [26, 33] and further supports the recommendation that the activPAL™ be considered the gold standard for assessment of free-living sedentary time [6], including in RA.
On the basis of this recommendation, we examined the validity of the RA-specific sedentary time count-based cut-point, using the activPAL3μ™ as the criterion standard. Results revealed a mean difference of 2.3 h/day between sedentary time quantified using the RA-specific count-based cut-point vs. the activPAL3μ™. Bland–Altman plots demonstrated most data points to fall above zero, suggesting overestimation of sedentary time using the RA-specific count-based cut-point, compared to the activPAL3μ™. Still, when compared to the activPAL3μ™, our RA-specific count-based cut-point produced a smaller mean difference, and narrower 95% LOA, relative to the commonly used non-RA count-based cut-point (< 100 counts/min) [11, 12].
It is possible that the observed lack of agreement between sedentary time quantified using the RA-specific count-based cut-point vs. activPAL3μ™-assessed sedentary time in this study reflects the inability of accelerometers to differentiate between sitting and standing, rather than relatively compromised validity of the RA-specific count-based cut-point described herein. Our data support this as a plausible explanation for two reasons. First, participants’ average MET value during ‘standing’ in the laboratory protocol was 0.8 METs (< the 1.5 METs used to define sedentary behaviour). Second, the downward trend observed in Bland–Altman plots suggests agreement between GT3X+- and activPAL3μ™-assessed sedentary time improves at higher levels of sedentary time, where lower levels of PA (including standing) are likely to occur. That is, for people engaging in high levels of sedentary time, standing may occupy less of daily waking behaviour and, therefore, there is less opportunity to misclassify standing time as sedentary time. In a recent study comparing accelerometer- and activPAL™-assessed sedentary time in older adults, Aguilar-Farías et al. [24] demonstrated that their population-specific sedentary time VM count-based cut-points (e.g., < 60 counts/min) were better able to detect combined activPAL™-assessed sedentary and standing time (AUC = 0.82), compared to activPAL™-assessed sedentary time alone (AUC = 0.73).
In summary, results suggest that future studies should employ the activPAL3μ™ for valid assessment of sedentary time in people living with RA. When this is not possible, the RA-specific sedentary time count-based cut-point represents a more valid alternative, relative to the non-RA count-based cut-point of < 100 counts/min [11, 12] in this population. However, these recommendations should be considered in the context of study limitations. First, the nature of the laboratory-validation meant that a free-living environment could not be wholly achieved, only replicated. Still, the laboratory protocol was informed by similar validation studies conducted in RA and non-RA populations, and included several activities typically undertaken in a free-living environment [21, 31, 34]. Second, participants not reaching steady-state VO2 during laboratory-validation activities were excluded from ROC curve analysis, which reduced the number of data points available for cut-point calibration (out of a possible 199: sedentary time = 82; LPA = 87; MPA = 30). Nevertheless, the number of data points for each activity intensity are comparable to other studies that have developed accelerometer count-based cut-points for measuring sedentary time, LPA and MPA in populations with reduced physical function [14]. Third, participants included in both laboratory- and field-based protocols were mostly females with moderate RA disease activity. Thus, findings may be less generalisable to male RA patients and those with more/less active disease. Future research should, therefore, confirm the validity of the RA-specific count-based cut-points and activPAL3μ™ in different populations of RA patients (e.g., males, higher/lower disease activity). The current study has provided a ‘first step’ towards further work in this area.
Finally, the primary aim of the current study was to develop RA-specific triaxial accelerometer count-based cut-points to allow researchers to easily and consistently apply these criteria to accelerometer data in the RA population with heightened accuracy, compared to non-RA (and uniaxial) count-based cut-points. Indeed, the development of RA-specific count-based cut-points fills an important gap in the literature, providing an accessible tool for the growing number of rheumatology professionals (e.g., consultants, nurses, physiotherapists) conducting research to understand the role of sedentary time and PA in RA. However, due to a rapidly evolving field and technological advancements in the measurement of sedentary time and PA, it is important that future research examines the validity of other emerging analytical approaches that involve the development of complex data processing algorithms, to compliment the count-based cut-point validation model employed herein.
Conclusion
This study confirms the activPAL3μ™ can be considered the gold standard for measurement of free-living sedentary time in RA. Further, RA-specific triaxial accelerometer count-based cut-points presented herein are sensitive and specific for measurement of sedentary time, LPA and MPA, and permit more accurate assessment of free-living sedentary time compared to the commonly employed non-RA uniaxial accelerometer count-based cut-point [11, 12]. Thus, in the absence of the activPAL3μ™, our data support use of the RA-specific count-based cut-point for assessment of sedentary time in this patient group.
References
Metsios GS, Kitas GD (2018) Physical activity, exercise and rheumatoid arthritis: effectiveness, mechanisms and implementation. Best Pract Res Clin Rheumatol 32(5):669–682
Sedentary Behaviour Research Network (2012) Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab 37:540–542
Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE et al (2017) Sedentary behavior research network (SBRN)—terminology consensus project process and outcome. Int J Behav Nutr Phys Act 14(1):75
Fenton SAM, Veldhuijzen van Zanten JJCS, Duda JL, Metsios GS, Kitas GD (2018) Sedentary behaviour in rheumatoid arthritis: definition, measurement and implications for health. Rheumatology(Oxford). 57(2):213–226
Verhoeven F, Tordi N, Prati C, Demougeot C, Mougin F, Wendling D (2016) Physical activity in patients with rheumatoid arthritis. Joint Bone Spine 83(3):265–270
Chastin SFM, Dontje ML, Skelton DA, Cukic I, Shaw RJ, Gill JMR et al (2018) Systematic comparative validation of self-report measures of sedentary time against an objective measure of postural sitting (activPAL). Int J Behav Nutr Phys Act 15(1):21
Healy GN, Clark BK, Winkler EA, Gardiner PA, Brown WJ, Matthews CE (2011) Measurement of adults’ sedentary time in population-based studies. Am J Prev Med 41(2):216–227
Sylvia LG, Bernstein EE, Hubbard JL, Keating L, Anderson EJ (2014) Practical guide to measuring physical activity. J Acad Nutr Diet 114(2):199–208
Semanik P, Song J, Chang RW, Manheim L, Ainsworth B, Dunlop D (2010) Assessing physical activity in persons with rheumatoid arthritis using accelerometry. Med Sci Sports Exerc 42(8):1493–1501
Arvidsson D, Fridolfsson J, Borjesson M (2019) Measurement of physical activity in clinical practice using accelerometers. J Intern Med 286(2):137–153
Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR et al (2008) Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol 167(7):875–881
Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M (2008) Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40(1):181–188
Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, Koutedakis Y, Nevill AM, Douglas KM et al (2008) New resting energy expenditure prediction equations for patients with rheumatoid arthritis. Rheumatology (Oxford) 47(4):500–506
Evenson KR, Wen F, Herring AH, Di C, LaMonte MJ, Tinker LF et al (2015) Calibrating physical activity intensity for hip-worn accelerometry in women age 60 to 91 years: The Women’s Health Initiative OPACH Calibration Study. Prev Med Rep 2:750–756
Heesch KC, Hill RL, Aguilar-Farias N, van Uffelen JGZ, Pavey T (2018) Validity of objective methods for measuring sedentary behaviour in older adults: a systematic review. Int J Behav Nutr Phys Act 15(1):119
Thomsen T, Aadahl M, Beyer N, Hetland ML, Loppenthin K, Midtgaard J et al (2017) The efficacy of motivational counselling and SMS reminders on daily sitting time in patients with rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis 76(9):1603–1606
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd et al (2010) 2010 Rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581
O’Brien CM, Duda JL, Kitas GD, Veldhuijzen van Zanten JJCS, Metsios GS, Fenton SAM (2019) Objective measurement of sedentary time and physical activity in people with rheumatoid arthritis: protocol for an accelerometer and activPAL validation study. Mediterr J Rheumatol 30(2):125–134
Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38(1):44–48
Fries JF, Spitz P, Kraines RG, Holman HR (1980) Measurement of patient outcome in arthritis. Arthritis Rheum 23(2):137–145
Copeland JL, Esliger DW (2009) Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act 17(1):17–30
Santos-Lozano A, Santin-Medeiros F, Cardon G, Torres-Luque G, Bailon R, Bergmeir C et al (2013) Actigraph GT3X: validation and determination of physical activity intensity cut points. Int J Sports Med 34(11):975–982
O’Brien CM, Duda JL, Kitas GD, Veldhuijzen van Zanten JJCS, Metsios GS, Fenton SAM (2018) Correlates of sedentary behaviour and light physical activity in people living with rheumatoid arthritis: protocol for a longitudinal study. Mediterr J Rheumatol. 29(2):106–117
Aguilar-Farías N, Brown WJ, Peeters GM (2014) ActiGraph GT3X+ cut-points for identifying sedentary behaviour in older adults in free-living environments. J Sci Med Sport 17(3):293–299
Pfister T, Matthews CE, Wang Q, Kopciuk KA, Courneya K, Friedenreich C (2017) Comparison of two accelerometers for measuring physical activity and sedentary behaviour. BMJ Open Sport Exerc Med 3(1):e000227
Edwardson CL, Winkler EAH, Bodicoat DH, Yates T, Davies MJ, Dunstan DW et al (2017) Considerations when using the activPAL monitor in field-based research with adult populations. J Sport Health Sci 6(2):162–178
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Giavarina D (2015) Understanding Bland Altman analysis. Biochem Med (Zagreb) 25(2):141–151
Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, Duda JL, Rouse PC, Yu CA et al (2017) Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 18(1):131
Fenton SAM, Veldhuijzen van Zanten JJCS, Metsios GS, Rouse PC, Yu CA, Kitas GD et al (2018) Autonomy support, light physical activity and psychological well-being in rheumatoid arthritis: a cross-sectional study. Ment Health Phys Act. 14:11–18
Larkin L, Nordgren B, Purtill H, Brand C, Fraser A, Kennedy N (2016) Criterion validity of the activPAL activity monitor for sedentary and physical activity patterns in people who have rheumatoid arthritis. Phys Ther 96(7):1093–1101
Dogan NO (2018) Bland–Altman analysis: a paradigm to understand correlation and agreement. Turk J Emerg Med 18(4):139–141
Sellers C, Dall P, Grant M, Stansfield B (2016) Validity and reliability of the activPAL3 for measuring posture and stepping in adults and young people. Gait Posture 43:42–47
Kim Y, Welk GJ (2015) Criterion validity of competing accelerometry-based activity monitoring devices. Med Sci Sports Exerc 47(11):2456–2463
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C et al (2011) 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 43(8):1575–1581
Funding
This research was undertaken as Doctoral research, supported in part, by the Medical Research Council-Versus Arthritis Centre for Musculoskeletal Ageing Research (CMAR) and by Russells Hall Hospital Charitable Research Fund.
Author information
Authors and Affiliations
Contributions
All authors were involved in forming the concept and research aims, and developing the methodology for this study. CM. O’Brien recruited participants, conducted data collections and managed the data. With input from JL. Duda, GD. Kitas and SAM. Fenton, CM. O’Brien applied statistical techniques to analyse the data collected. CM. O’Brien prepared and wrote the initial draft of this manuscript, with reviews and revision undertaken by all authors. All authors read and approved the final manuscript. JL. Duda, GD. Kitas and SAM. Fenton acquired the financial support for this study leading to this publication. SAM. Fenton was the Principal Investigator for this study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Black Country (West Midlands) Research Ethics Committee (16/WM/0371).
Consent to participate
Informed consent was obtained from all participants included in the study.
Disclaimer
The protocol of this study has previously been published (O’Brien et al. [18]). As a result, some of the text included herein may duplicate information reported in the prior publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Brien, C.M., Duda, J.L., Kitas, G.D. et al. Measurement of sedentary time and physical activity in rheumatoid arthritis: an ActiGraph and activPAL™ validation study. Rheumatol Int 40, 1509–1518 (2020). https://doi.org/10.1007/s00296-020-04608-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04608-2