Abstract
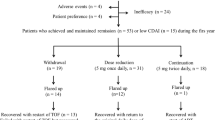
The current strategy for managing rheumatoid arthritis (RA) focuses on achieving clinical remission. Once remission is achieved and sustained over time, the most efficient strategy is dose optimization. This work describes the results of dose optimization after 2 years of follow-up in patients with RA treated with biological therapy and identifies predictive variables of response. Cohort: patients from the CREATE registry who, as of 1 November 2013, had been in clinical remission (DAS28 ≤2.6) for at least 6 months. Intervention: Dose optimization was 20–50% of the standard dose. Outcome measurement were effectiveness (percentage of patients who continued to meet criteria for clinical remission) and efficiency (dose reduction and mean savings). Sixty-eight patients with RA were optimized, with initial mean DAS28 of 2.2 ± 0.7. After 2 years of follow-up, the mean DAS28 was 2.4 ± 0.7, a non-statistically significant difference. Twenty-eight patients (41.2%) continued in clinical remission with dose optimization after 2 years. Mean survival time was 14.2 months (95% CI 12.0–16.5). Of the 40 patients who needed to return to a standard dose, 57.5% managed to achieve remission again at 2 years. Mean dose reduction at 2 years was 21.17%, reaching a mean saving of €5576 ± 5099 per patient. In actual clinical practice, over 40% of patients with established RA who had been in sustained clinical remission managed to continue in remission 2 years after receiving optimized doses. The savings achieved was about 21% of the actual direct health costs for patients in the CREATE registry.


Similar content being viewed by others
References
Sanmartí R, García-Rodríguez S, Álvaro-Gracia JM, Andreu JL, Balsa A, Cáliz R et al (2015) Actualización 2014 del Documento de Consenso de la Sociedad Española de Reumatología sobre el uso de terapias biológicas en la artritis reumatoide. Reumatol Clin 11:279–294
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M et al (2013) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73:492–509
Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT et al (2010) Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 62:22–32
Leffers HC, Ostergaard M, Glintborg B, Krogh NS, Foged H, Tarp U et al (2011) Efficacy of abatacept and tocilizumab in patients with rheumatoid arthritis treated in clinical practice: results from the nationwide Danish DANBIO registry. Ann Rheum Dis 70:1216–1222
Maneiro JR, Pérez-Pampin E, Salgado E, Carmona L, Gómez-Reino JJ (2014) Observational study of optimization of biologic therapies in rheumatoid arthritis: a single-centre experience. Rheumatol Int 34:1059–1063
Smolen JS, Nash P, Durez P, Hall S, Ilivanova E, Irazoque-Palazuelos F et al (2013) Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 381:918–929
Smolen JS, Emery P, Fleischmann R, van Hollenhoven R, Pavelka K, Durez P et al (2014) Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 383:321–332
Brocq O, Millasseau E, Albert C, Grisot C, Flory P, Roux CH et al (2009) Effect of discontinuing TNFalpha antagonist therapy in patients with remission of rheumatoid arthritis. Joint Bone Spine 76:350–355
Van Den Broek M, Klarenbeek NB, Dirven L, Dirven L, van Schaardenburg D, Hulsmans HM et al (2011) Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score-steered therapy: subanalysis of the Best study. Ann Rheum Dis 70:1389–1394
Tanaka Y, Takeuchi T, Mimori T, Saito K, Nawata M, Kameda H et al (2010) Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis 69:1286–1291
Van Der Kooij SM, Goekoop-Ruiterman YP, De Vries-Bouwstra JK, Guler-Yuksel M, Zwinderman AH, Kerstens PJ et al (2009) Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis 68:914–921
Saleem B, Keen H, Goeb V, Parmar R, Nizam S, Hensor EM et al (2010) Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped? Ann Rheum Dis 69:1636–1642
Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC et al (2000) A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 343:1586–1593
Fautrel B, Pham T, Alfaite T, Gandjbakhch Foltz V, Morel J et al (2016) Step-down strategy of spacing TNF-blocker injections for established rheumatoid arthritis in remission: results of the multicentre non-inferiority randomised open-label controlled trial (STRASS: spacing of TNF-blocker injections in Rheumatoid ArthritiS Study). Ann Rheum Dis 75:59–67
González-Alvaro I, Martínez-Fernández C, Dorantes-Calderón B, García-Vicuña R, Hernández-Cruz B, Herrero-Ambrosio A et al (2015) Spanish Rheumatology Society and Hospital Pharmacy Society Consensus on recommendations for biologics optimization in patients with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis. Rheumatology (Oxford) 54:1200–1209
Cárdenas M, de la Fuente S, Castro-Villegas MC, Romero-Gómez M, Ruiz-Vílchez D, Calvo-Gutiérrez J et al (2016) Cost-effectiveness of clinical remission by treat to target strategy in established rheumatoid arthritis: results of the CREATE registry. Rheumatol Int 36:1627–1632
Prevoo MLL, Van‘T Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38:44–48
Den Broeder AA, Van Der Maas A, Van Den Bemt BJF (2010) Dose de-escalation strategies and role of therapeutic drug monitoring of biologics in RA. Editorial. Rheumatology 49:1801–1803
Smolen JS, Alehata D (2015) Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol 11:276–289
Van Vollehoven R, Ostegaard M, Leirisalo-Repo M, Uhlig T, Jansson M, Larsson E et al (2016) Full dose, reduced dose or discontinuation of etanercept in rheumatoid arthritis. Ann Rheum Dis 75:52–58
Kievit W, Van Herwaarden N, Van Den Hoogen FH, van Vollenhoven RF, Bijlsma JW, van den Bemt BJ et al (2016) Disease activity-guided dose optimisation of adalimumab and etanercept is a cost-effective strategy compared with non-tapering tight control rheumatoid arthritis care: analyses of the DRESS study. Ann Rheum Dis 75:1939–1944
Turchetti G, Scalone L, Della Casa Alberighi O, Mosca M, Montella S, Cortesi PA et al (2012) The rationale of pharmacoeconomic analysis in rheumatologic indications. Clin Exp Rheumatol 30:S64–S71
Cárdenas M, De La Fuente S, Font P, Castro-Villegas MC, Romero-Gómez M, Ruiz-Vílchez D et al (2016) Real-world cost-effectiveness of infliximab, etanercept and adalimumab in rheumatoid arthritis patients: results of the CREATE registry. Rheumatol Int 36:231–241
Acknowledgments
No funding has been received to carry out this study or for preparation of the manuscript. The authors thank Mª Dolores Aguilar-Conesa for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study meets the standards of Good Clinical Practice, the principles of the Declaration of Helsinki and Order SAS 347/2009 of December 16, which develops guidelines on observational post-authorization studies for drugs used in humans in Spain. Patient data are coded to maintain anonymity in the study and to prevent their identification by third parties. The study was approved by the Ethical Committee of the Reina Sofia University Hospital of Cordoba.
Conflict of interest
Cárdenas M, Font P, Castro-Villegas and Collantes-Estévez E, report grants, consulting fees, or lecture fees from MSD, Pfizer or AbbVie, none of which were related to the present work. De la Fuente S, Romero-Alonso M, Calvo-Gutiérrez J, Escudero-Contreras A and Del Prado JR have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cárdenas, M.J., de la Fuente, S., Castro-Villegas, M.C. et al. Optimization of biological therapy in rheumatoid arthritis patients: outcomes from the CREATE registry after 2 years of follow-up. Rheumatol Int 37, 1701–1708 (2017). https://doi.org/10.1007/s00296-017-3757-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3757-7