Abstract
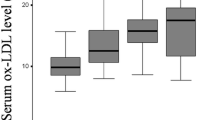
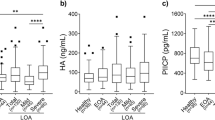
We investigated serum prolidase activity and oxidative/antioxidative status in patients with knee osteoarthritis (OA) and evaluated its relationships with radiographic severity and clinical parameters. The study population consisted of 137 patients with knee OA and 134 healthy volunteers. The severity of knee OA was classified according to the Kellgren–Lawrence criteria. Each patient was also evaluated clinically according to the Western Ontario and McMaster University Osteoarthritis Index (WOMAC). Serum prolidase activity was measured spectrophotometrically. Oxidative status was assessed by measuring serum lipid hydroperoxide (LOOH) and total oxidative status (TOS). Antioxidative status was assessed by measuring serum-free sulfhydryl groups (–SH = total thiol) and total antioxidant capacity (TAC). Oxidative stress index (OSI) was calculated. Serum prolidase activity was significantly lower in the knee OA group than in the control group (p < 0.001). The serum prolidase activities decreased with the severity of knee OA. Furthermore, serum LOOH, TOS, and OSI levels of the knee OA group were significantly higher than those of the controls (p < 0.001 for all), whereas TAC and –SH levels did not differ between the two groups (p > 0.05). In a multiple regression analysis, WOMAC score was independently associated with serum prolidase activity (β = −0.340, p < 0.001). Decreased serum prolidase activity and elevated LOOH, TOS, and OSI levels may be associated with knee OA, and serum prolidase activity may be a useful adjunctive indicator of the progression of knee OA in follow-up.


Similar content being viewed by others
References
Ertürk C, Altay MA, Selek S, Koçyiğit A (2012) Paraoxonase-1 activity and oxidative status in patients with knee osteoarthritis and their relationship with radiological and clinical parameters. Scand J Clin Lab Invest 72(5):433–439
Brandt KD, Dieppe P, Radin EL (2009) Commentary: Is it useful to subset “primary” osteoarthritis? A critique based on evidence regarding the etiopathogenesis of osteoarthritis. Semin Arthritis Rheum 39(2):81–95
Ertürk C, Altay MA, Altay N, Kalender AM, Oztürk IA (2014) Will a single periarticular lidocaine-corticosteroid injection improve the clinical efficacy of intraarticular hyaluronic acid treatment of symptomatic knee osteoarthritis? Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-014-3398-2
Miltyk W, Surazynski A, Kasprzak KS, Fivash MJ Jr, Buzard GS, Phang JM (2005) Inhibition of prolidase activity by nickel causes decreased growth of proline auxotrophic CHO cells. J Cell Biochem 94(15):1210–1217
Surazynski A, Miltyk W, Palka J, Phang JM (2008) Prolidase-dependent regulation of collagen biosynthesis. Amino Acids 35(4):731–738
Altay MA, Erturk C, Aksoy N, Taskin A, Bilge A, Celik H, Isikan UE (2011) Serum prolidase activity and oxidative-antioxidative status in Legg–Calve–Perthes disease. J Pediatr Orthop B 20(4):222–226
Altay MA, Erturk C, Aksoy N, Taskın A, Isıkan UE (2011) A preliminary study pointing out the role of serum prolidase activity and oxidative-antioxidative status parameters during the treatment process of patients with idiopathic clubfoot. Scand J Clin Lab Invest 71(7):576–582
Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M (2007) Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int 27(4):339–344
Ziskoven C, Jäger M, Zilkens C, Bloch W, Brixius K, Krauspe R (2010) Oxidative stress in secondary osteoarthritis: From cartilage destruction to clinical presentation? Orthop Rev (Pavia) 2(2):e23
Davies CM, Guilak F, Weinberg JB, Fermor B (2008) Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthr Cartil 16(5):624–630
Martel-Pelletier J, Pelletier JP (2010) Is osteoarthritis a disease involving only cartilage or other articular tissues? Eklem Hastalik Cerrahisi 21(1):2–14
Loeser RF (2011) Aging and osteoarthritis. Curr Opin Rheumatol 23(5):492–496
Kim J, Xu M, Xo R, Mates A, Wilson GL, Pearsall AW 4th, Grishko V (2010) Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthr Cartil 3:424–432
Brandl A, Hartmann A, Bechmann V, Graf B, Nerlich M, Angele P (2011) Oxidative stress induces senescence in chondrocytes. J Orthop Res 29(7):1114–1120
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American Rheumatism Association. Arthritis Rheum 29(8):1039–1049
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthrosis. Ann Rheum Dis 16:494–502
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840
Ozcan O, Gultepe M, Ipcioglu O, Bolat B, Kayadibi H (2007) Optimization of the photometric enzyme activity assay for evaluating real activity of prolidase. Turk J Biochem 32:12–16
Myara I, Charpentier C, Lemonnier A (1982) Optimal conditions for prolidase assay by proline colorimetric determination: application to iminodipeptiduria. Clin Chim Acta 125:193–205
Nourooz-Zadeh J (1999) Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol 300:58–62
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Hu ML, Louie S, Cross CE, Motchnik P, Halliwell B (1993) Antioxidant protection against hypochlorous acid in human plasma. J Lab Clin Med 121:257–262
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Erel O (2004) A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 37:112–119
Gencer M, Aksoy N, Dagli EC, Uzer E, Aksoy S, Selek S, Celik H, Cakir H (2011) Prolidase activity dysregulation and its correlation with oxidative-antioxidative status in chronic obstructive pulmonary disease. J Clin Lab Anal 25(1):8–13
Evrenkaya TR, Atasoyu EM, Kara M, Unver S, Gultepe M (2006) The role of prolidase activity in the diagnosis of uremic bone disease. Ren Fail 28(4):271–274
Sezen Y, Bas M, Altiparmak H, Yildiz A, Buyukhatipoglu H, Faruk Dag O, Kaya Z, Aksoy N (2010) Serum prolidase activity in idiopathic and ischemic cardiomyopathy patients. J Clin Lab Anal 24(4):213–218
DeGroot J, Verzijl N, Jacobs KM, Budde M, Bank RA, Bijlsma JW, TeKoppele JM, Lafeber FP (2001) Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthr Cartil 9(8):720–726
Langberg H, Skovgaard D, Asp S, Kjaer M (2000) Time pattern of exercise-induced changes in type I collagen turnover after prolonged endurance exercise in humans. Calcif Tissue Int 67(1):41–44
Yudoh K, Nguyen T, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K (2005) Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther 7(2):380–391
Bedson J, Croft PR (2008) The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 9:116
Ishijima M, Watari T, Naito K, Kaneko H, Futami I, Yoshimura-Ishida K, Tomonaga A, Yamaguchi H, Yamamoto T, Nagaoka I, Kurosawa H, Poole RA, Kaneko K (2011) Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther 13(1):22
Martin KR, Kuh D, Harris TB, Guralnik JM, Coggon D, Wills AK (2013) Body mass index, occupational activity, and leisure-time physical activity: an exploration of risk factors and modifiers for knee osteoarthritis in the 1946 British birth cohort. BMC Musculoskelet Disord 14:219. doi:10.1186/1471-2474-14-219
Ertürk C, Altay MA, Sert C, Levent A, Yaptı M, Yüce K (2015) The body composition of patients with knee osteoarthritis: relationship with clinical parameters and radiographic severity. Aging Clin Exp Res. doi:10.1007/s40520-015-0325-4
Acknowledgments
Approval of local ethics committee reference number: B.30.2.HRÜ0.0.20.05.00.050.01.04-90. The authors declare that they have no relevant financial involvement with any commercial organization with direct financial interest in the subject or materials discussed in this manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altay, M.A., Ertürk, C., Bilge, A. et al. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: relationships with radiographic severity and clinical parameters. Rheumatol Int 35, 1725–1731 (2015). https://doi.org/10.1007/s00296-015-3290-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-015-3290-5